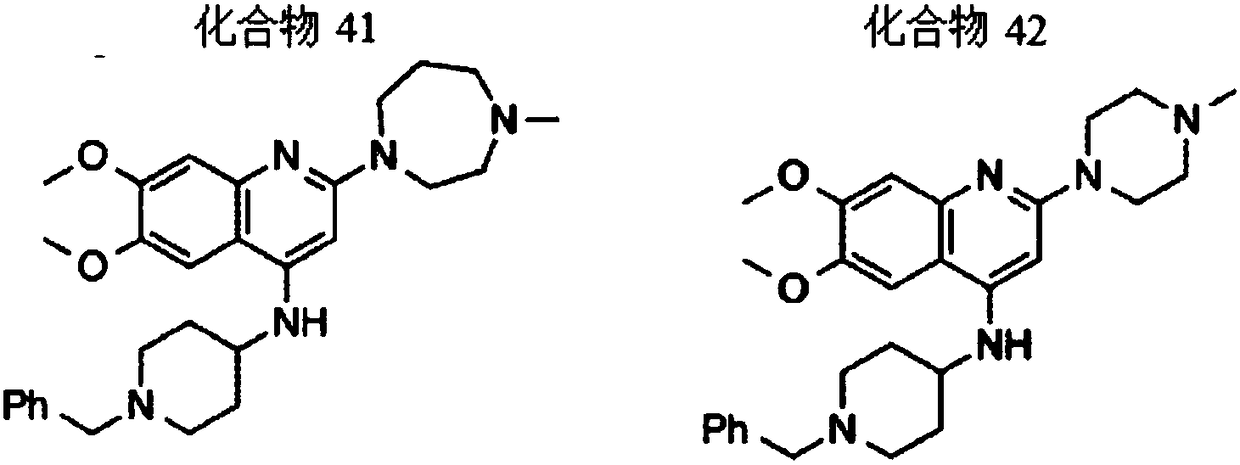
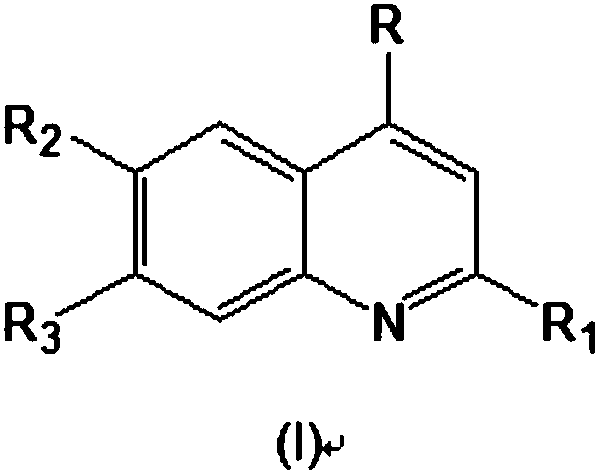
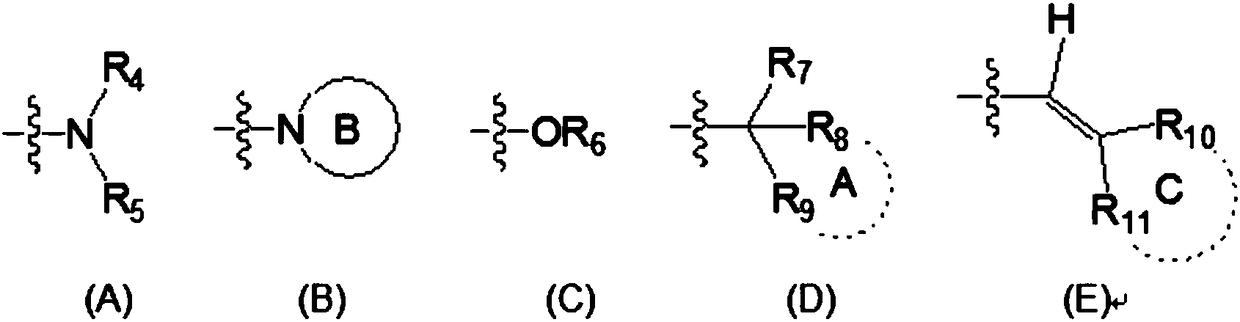
2,4,6,7-tetrasubstituted quinoline compounds as inhibitors of DNA methyltransferases
A compound and substituent technology, applied in the field of 2,4,6,7-quaternary substituted quinoline compounds, can solve problems such as inactivity and achieve effective therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0326] The general procedure of the preparative HPLC purification method:
[0327] HPLC measurements were performed using a Gilson 281 from a 233 pump (binary), autosampler and UV detector. Fractions were detected by LC-MS. The MS detector was configured with an electrospray ionization source. The source temperature was maintained at 300-350°C.
[0328] HPLC method (purification method):
[0329] General conditions for methods 1~2, 5~14, 17~42: Reverse phase HPLC was performed on a Luna C18 (100 x 30 mm; 5 μm). Solvent A: water containing 0.1% trifluoroacetic acid; solvent B: acetonitrile. UV detector.
[0330] General conditions for methods 3 and 15: Reverse phase HPLC was performed on a Luna C18 (100 x 30 mm; 4 μm). Solvent A: water containing 0.075% trifluoroacetic acid; solvent B: acetonitrile containing 0.075% trifluoroacetic acid. UV detector.
[0331] General conditions for Method 4: Reverse phase HPLC was performed on a Waters Xbridge (150 x 25 mm; 5 μm)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com