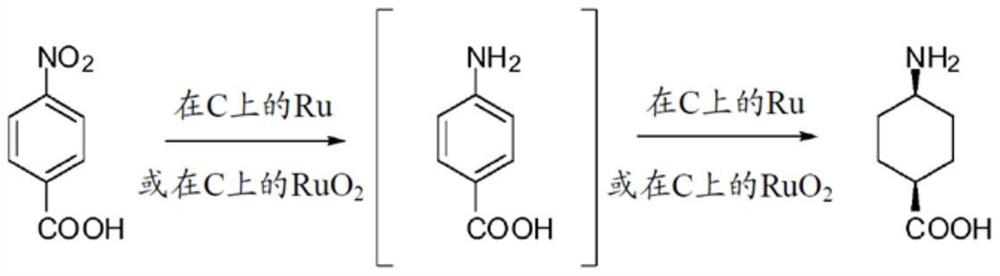
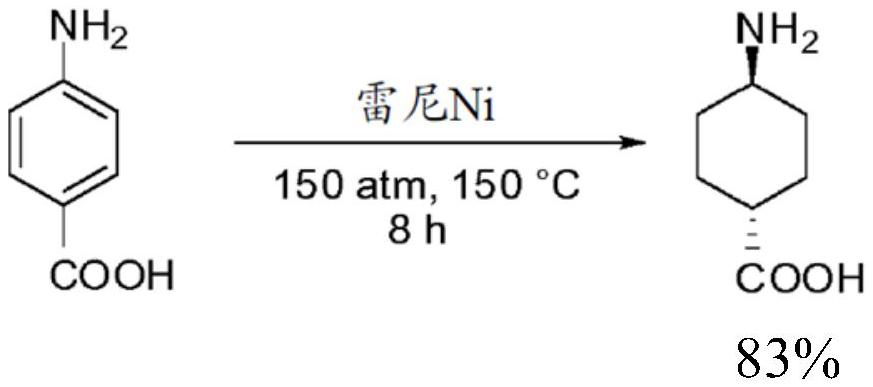
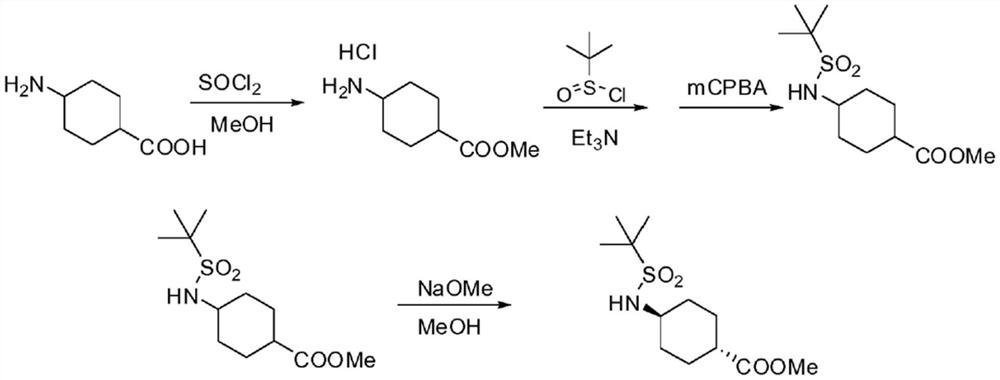
Method for preparing trans-4-amino-1-cyclohexane carboxylic acid and derivatives thereof
A reaction and compound technology, applied in the field of preparation of trans-4-amino-1-cyclohexanecarboxylic acid derivatives, can solve problems such as spontaneous combustion behavior, reduced attractiveness of industrial production, and difficulty in isolating products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of 4-aminocyclohexane-1-carboxylic acid (cis / trans mixture) (1):
[0060] p-Aminobenzoic acid (10.0 g, 0.07 mol, 1 equiv), 5% Ru / C (2.50 g) and 10% NaOH (100.0 mL) were mixed in an autoclave. The mixture was stirred at 100° C. and 15 bar of hydrogen. After stirring for 20 hours, no starting material was observed on TLC (DCM / MeOH / NH 3 =5 / 5 / 1 (v / v / v); stain: ninhydrin). Complete conversion and cis:trans ratio = 1:4.6 according to NMR. Stop responding.
[0061] The table below gives a summary of the examples carried out according to the method described in Example 1.
[0062]
Embodiment 2
[0064] Preparation of 4-{[(tert-butoxy)carbonyl]amino}cyclohexane-1-carboxylic acid (cis / trans mixture) (2)
[0065] The catalyst from Example 1 was unfiltered. Acetone (327.3 mL, 30 volumes) and Boc anhydride (16.63 g, 0.07 mol, 1.0 equiv) were added. The reaction mixture was stirred at room temperature for 20 hours. Thereafter, TLC control (DCM / MeOH / NH 3 = 5 / 5 / 1 (v / v / v), lead to KMnO 4 (product sensitive) and ninhydrin (substrate sensitive)) only showed product, so the reaction was complete. The catalyst was filtered through celite and washed with 600 mL of the mixture (acetone / water=4 / 1 (v / v)). Acetone was evaporated under reduced pressure. The aqueous layer (pH=9) was extracted 3 times with dichloromethane (DCM) (3 x 75 mL). The aqueous solution was acidified to pH = 4 with 20 g of citric acid and extracted 5 times with DCM (5 x 100 mL). The combined organic layers were washed with Na 2 SO 4 After drying and filtering, the organic solution was evaporated and dried...
Embodiment 3
[0067] Isolation of trans-4-{[(tert-butoxy)carbonyl]amino}cyclohexane-1-carboxylic acid from its cis derivative
[0068] BOC-amino acid ((2) 12.99g, 0.05mol, 1.0 equivalent, cis: trans=1:3.6) and K 2 CO 3 (2 g, 0.06 mol, 1.2 eq relative to the cis content in (2)) were mixed and suspended in acetone (259 mL, 20.0 vol). Methyl bromide (1.93 mL, 2.82 g, 0.02 mol, 0.43 equiv (2.0 equiv calculated for the cis isomer content in the mixture)) was added to the reaction mixture with stirring. The reaction mixture was stirred at 60 °C for 3 hours. A white precipitate appeared. The reaction mixture was cooled to room temperature over 30 minutes, then stirred at -10°C for 1 hour. After this time, the precipitate was filtered and washed with 100 mL of acetone (cooled to -10°C). The precipitate was added to 200 mL of 20% citric acid and 100 mL of DCM. Multiple layers are separated. The aqueous layer was extracted 4 times with DCM (4 x 100 mL). The combined organic layers were washed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com