Efficient iron series catalyst for catalyzing isoprene polymerization as well as preparation method and application thereof
A technology of isoprene and catalyst, which is applied in the direction of iron organic compounds and iron group organic compounds without C-metal bonds, etc., can solve the problems of high cost, insensitivity, and wide molecular weight distribution, and achieve low cost and high molecular weight distribution. Narrow, high molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
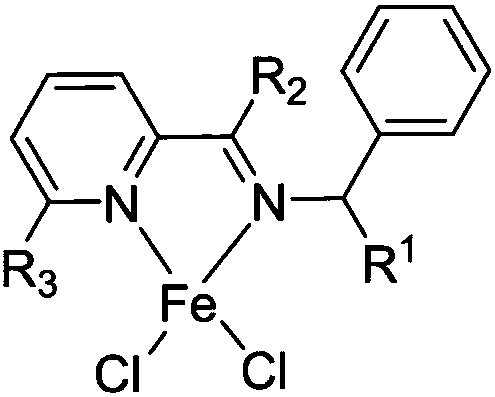
[0049] The present embodiment prepares the pyridine imine iron complex shown in formula (1):
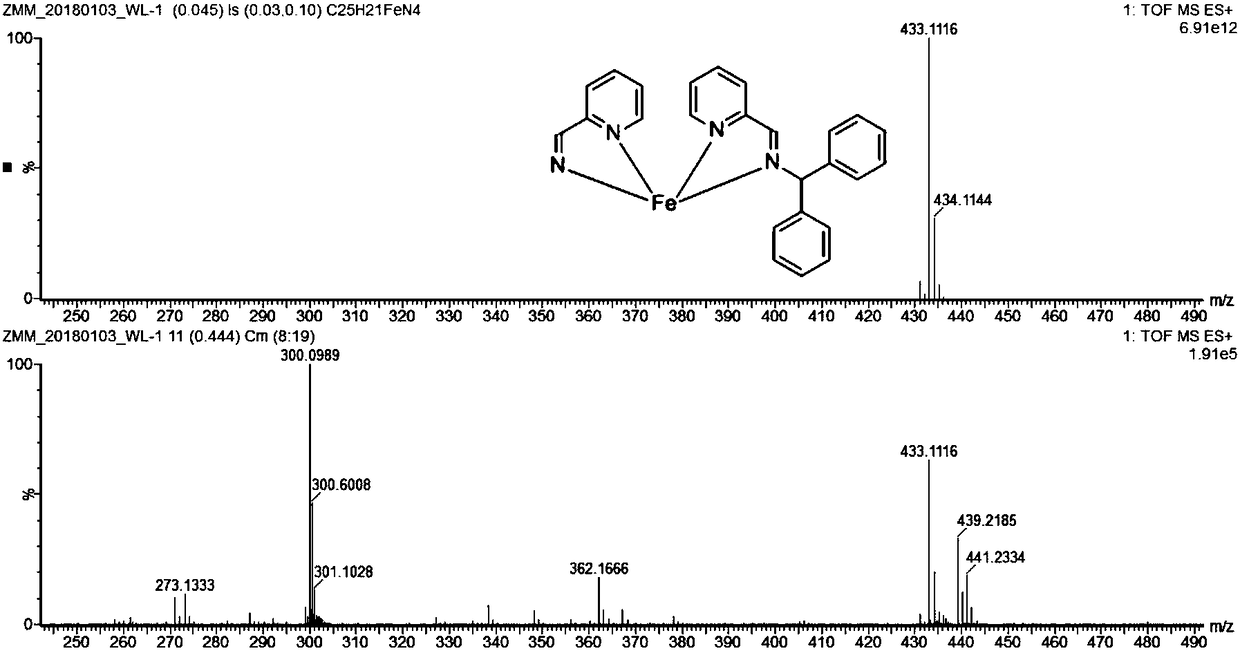
[0050] The 25mL Schlenk reaction tube was pumped and baked three times, and 15mL redistilled dichloromethane, equimolar ratio of anhydrous FeCl 2 And benzyl-substituted pyridinimine ligand, stirred at room temperature for 24h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain 340 mg of purple solid (85% yield).
[0051] Mass Spectrometry: C 13 h 12 ClFeN 2 [M-Cl] + : Theoretical value: 287.0038; measured value: 287.0031.
[0052] Elemental Analysis: C 13 h 12 Cl 2 FeN 2 : Theoretical value: C, 48.34%; H, 3.74%; N, 8.67%; Found value: C, 48.11%; H, 3.82%; N, 8.55%.
[0053] Magnetic susceptibility: (500MHz, CD 2 Cl 2 ):μ eff =5.32μ B (8.50 mg / mL).
[0054] H NMR spectrum: 1 H NMR (500MHz, CD 2 Cl 2 ,40℃,δ):95.8(Δ ν1 / 2 =460Hz), 68.8(Δ...
Embodiment 2
[0056] The preparation process of the pyridinium iron complex shown in formula (2) prepared in this embodiment is as follows:
[0057] The 25mL Schlenk reaction tube was pumped and baked three times, and 10mL redistilled dichloromethane, anhydrous FeCl 2 And methyl phenyl substituted pyridine imine ligand, stirred at room temperature for 48h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL distilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain 208 mg of blue-purple solid (>99% yield).
[0058] Mass Spectrometry: C 14 h 14 ClFeN 2 [M-Cl] +: Theoretical value: 301.0189; Measured value: 301.0189
[0059] Elemental Analysis: C 14 h 14 N 2 Cl 2 Theoretical Fe: C, 49.89%; H, 4.19%; N, 8.31%; Found: C, 49.70%; H, 3.96%; N, 8.21%.
[0060] Magnetic susceptibility (500MHz, CD 2 Cl 2 ):μ eff =4.98μ B (7.9mg / mL).
[0061] H NMR spectrum: 1 H NMR (500MHz, CD 2 Cl 2 ,40℃,δ):91.1(Δ ν1 / 2 =477Hz...
Embodiment 3
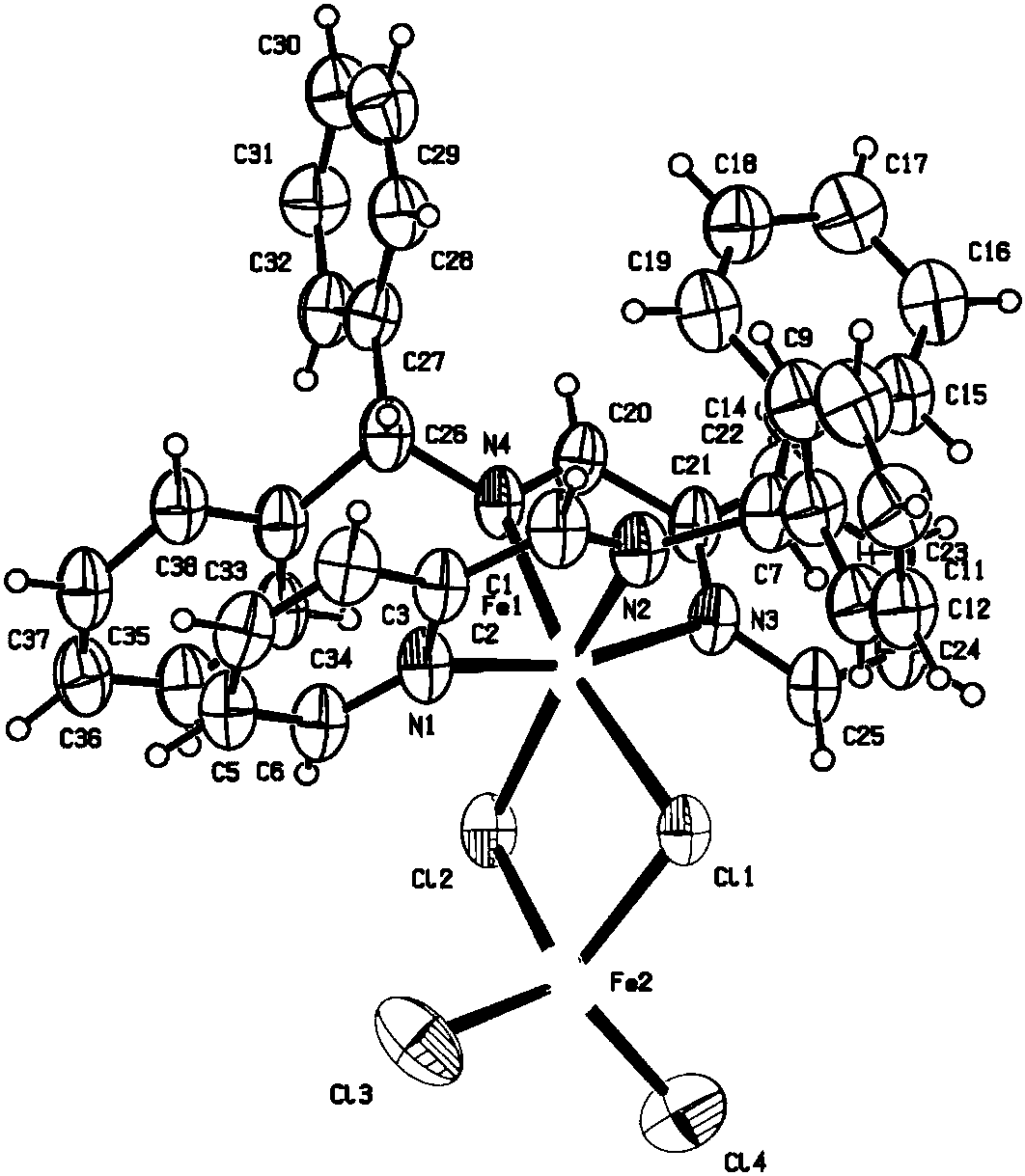
[0063] The pyridine imine iron complex shown in the formula (3) prepared in this embodiment, the preparation process is as follows:
[0064] The 10mL Schlenk reaction tube was pumped and baked three times, and 5mL redistilled dichloromethane, anhydrous FeCl 2 And diphenyl substituted pyridine imine ligand, stirred at room temperature for 48h. After the reaction, dichloromethane was vacuum-dried, washed twice with 10 mL redistilled n-hexane (the filtrate was colorless and clear), and vacuum-dried to constant weight to obtain 170 mg of red-purple solid (85% yield).
[0065] Mass Spectrometry: C 38 h 32 ClFeN 4 [M-FeCl 3 ] + : Theoretical value: 635.1659; measured value: 635.1658.
[0066] Elemental Analysis: C 38 h 32 Cl 4 Fe 2 N 4 Theoretical: C, 57.18%; H, 4.04%; N, 7.02%; Found: C, 57.26%; H, 4.10%; N, 7.01%.
[0067] Magnetic susceptibility (500MHz, CD 2 Cl 2 ):μ eff =5.32μ B (8.50mg / mL).
[0068] H NMR spectrum: 1 H NMR (500MHz, CD 2 Cl 2 ,40℃,δ):82.2(Δ ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com