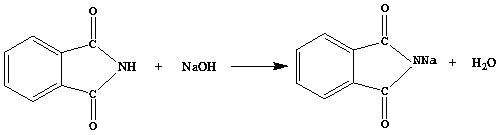N-(2-mercaptobenzothiazole) phthalimide and preparation method and application thereof
A technology of phthalimide and phthalimide salt, applied in the field of new compound, N-(2-mercaptobenzothiazole) phthalimide and its preparation, capable of Solve the problems of insufficient scorch time, general anti-scorch performance, and few varieties and quantities of multi-functional rubber additives, and achieve good scorch safety, rapid vulcanization performance, white appearance, and prolonged scorch time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Inject 21.30g of chlorine gas into a container containing 25.05g of 2-mercaptobenzothiazole (the molar ratio of chlorine gas to 2-mercaptobenzothiazole is 2.0:1), 200.4g of methyl silicone oil (the 2-mercaptobenzothiazole The mass ratio of thiazole to organic solvent is 1:8), and the closed and chlorination reaction is carried out at a temperature of 120 ° C. The total reaction time is 3 hours, and the reaction liquid of 2-sulfenyl chloride benzothiazole is obtained , with a purity of 99.6%;
[0035] 2. Add 14.7g of phthalimide and 13.13g of 32% sodium hydroxide solution (the molar ratio of phthalimide and sodium hydroxide is 1:1.05) into the reactor , stirred at low temperature for 2 hours at 10°C to obtain a reaction solution of sodium phthalimide with a purity of 99.4%;
[0036] 3. the reaction solution of the above-mentioned prepared sodium phthalimide and 2-sulfenyl chloride benzothiazole according to the mol ratio of phthalimide sodium and 2-sulfenyl chloride ...
Embodiment 2
[0039]1. Pass chlorine gas into the container filled with 2-mercaptobenzothiazole (the molar ratio of chlorine gas and 2-mercaptobenzothiazole is 1.8:1), C 5 -C 9 In the still body of paraffin solvent oil (the mass ratio of 2-mercaptobenzothiazole and organic solvent is 1:5), the airtight and chlorination reaction is carried out at a temperature of 150°C, and the total reaction time is 2h , to obtain the reaction solution of 2-sulfenyl chloride benzothiazole, the purity is 98.5%;
[0040] 2. Add phthalimide and 20% sodium hydroxide solution into the reaction kettle according to the molar ratio of 1:1, stir and react at 0°C for 3 hours to obtain phthalimide The reaction solution of sodium amine, the purity is 99.1%;
[0041] 3. the reaction solution of the above-mentioned prepared sodium phthalimide and 2-sulfenyl chloride benzothiazole according to the mol ratio of phthalimide sodium and 2-sulfenyl chloride benzothiazole Mixed at a ratio of 1:1.0, stirred and reacted at 0°C...
Embodiment 3
[0044] 1. Pass chlorine gas into a container containing 2-mercaptobenzothiazole (the molar ratio of chlorine gas to 2-mercaptobenzothiazole is 2.5:1), methyl silicone oil (the mass of 2-mercaptobenzothiazole and organic solvent In the still body with a ratio of 1:10), the airtight and chlorination reaction was carried out at a temperature of 80°C. The total reaction time was 4 hours, and the reaction liquid of 2-sulfenyl chloride benzothiazole was obtained with a purity of 98.9%;
[0045] 2. Add phthalimide and 32% sodium hydroxide solution into the reaction kettle at a molar ratio of 1:1.05, and stir at 20°C for 1 hour to obtain phthalimide The reaction solution of sodium amine, the purity is 99.3%;
[0046] 3. the reaction solution of the above-mentioned prepared sodium phthalimide and 2-sulfenyl chloride benzothiazole according to the mol ratio of phthalimide sodium and 2-sulfenyl chloride benzothiazole Mixed at a ratio of 1:1.5, stirred and reacted at 20°C for 2 hours at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com