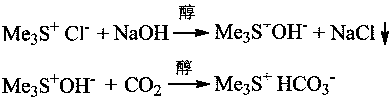Synthesis method for trimethyl-sulfonium-hydrocarbonate
A technology of trimethylsulfonium bicarbonate and synthesis method, applied in the direction of organic chemistry, which can solve the problems of high environmental protection treatment pressure, high cost of raw materials, high toxicity of by-products, etc., to reduce production energy consumption, facilitate transportation and storage, and react and transform high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
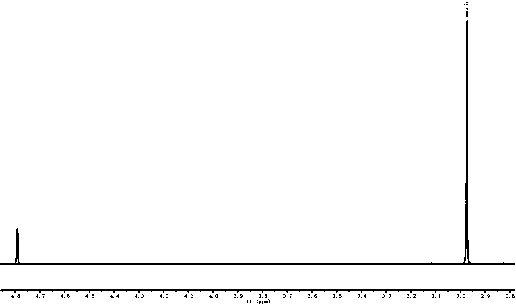
[0021] Embodiment one: 2.05 g of trimethylsulfonium chloride salts are dissolved in 15 mL of absolute ethanol, cooled and stirred in an ice-water bath, slowly dropwise add 10 mL of ethanol solution containing 0.80 g of sodium hydroxide, after the dropwise addition, continue to maintain for 10 Stir the reaction at ~15°C for 0.5h, let it stand for 1.0h, and filter to remove sodium chloride. Slowly inject 0.98 g of carbon dioxide gas into the filtrate until the pH value of the reaction solution is about 8.2, and recover ethanol by rotary evaporation under reduced pressure at 45°C. Obtain 2.37 g target product trimethylsulfonium bicarbonate salt, productive rate is 94.2%, 1 HNMR (D 2 O,600M): 2.98 (s.9H), the typical proton nuclear magnetic resonance spectrum of the product is attached figure 1 , this product can be used directly for subsequent applications.
Embodiment 2
[0022] Example 2: Dissolve 11.26g of trimethylsulfonium chloride salt in 50mL of anhydrous methanol, cool and stir in an ice-salt bath at -5°C, and slowly add dropwise 30mL of methanol solution containing 4.02g of sodium hydroxide. After the dropwise addition, Continue to maintain stirring reaction at 0~5°C for 2.0h, filter to remove sodium chloride, slowly pass 6.6 g of carbon dioxide gas into the filtrate until the pH value of the reaction solution is about 7.5, and recover methanol by rotary evaporation at 40°C to obtain 13.28 g The target product, trimethylsulfonium bicarbonate salt, has a yield of 96.1%. This product can be used directly for subsequent applications, and the crude product can also be reprocessed with anhydrous isopropanol to obtain higher purity trimethylsulfonium bicarbonate salt.
Embodiment 3
[0023] Example 3: Dissolve 11.25g of trimethylsulfonium chloride salt in 75mL of anhydrous isopropanol, stir in a water bath at room temperature, slowly add dropwise 30mL of methanol solution containing 4.02g of sodium hydroxide, after the dropwise addition, continue to stir After reacting for 1.0 h, remove sodium chloride by filtration, slowly pass through the filtrate with 6.0 g of carbon dioxide gas until the pH value of the reaction solution is about 8.0, and then recover the alcohol solvent by rotary evaporation under reduced pressure at 45 °C to obtain 12.82 g of the target product trimethyl base sulfonium bicarbonate salt, the productive rate is 92.8%, and this product can directly carry out follow-up application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com