Application of ultra-microporous metal-organic framework materials in isotope separation of hydrogen
A technology of isotope separation and organic framework, applied in the field of nuclear energy utilization, can solve the problems of inability to large-scale application, low separation efficiency, low separation efficiency, etc., and achieve the effects of cheap synthetic raw materials, low price, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
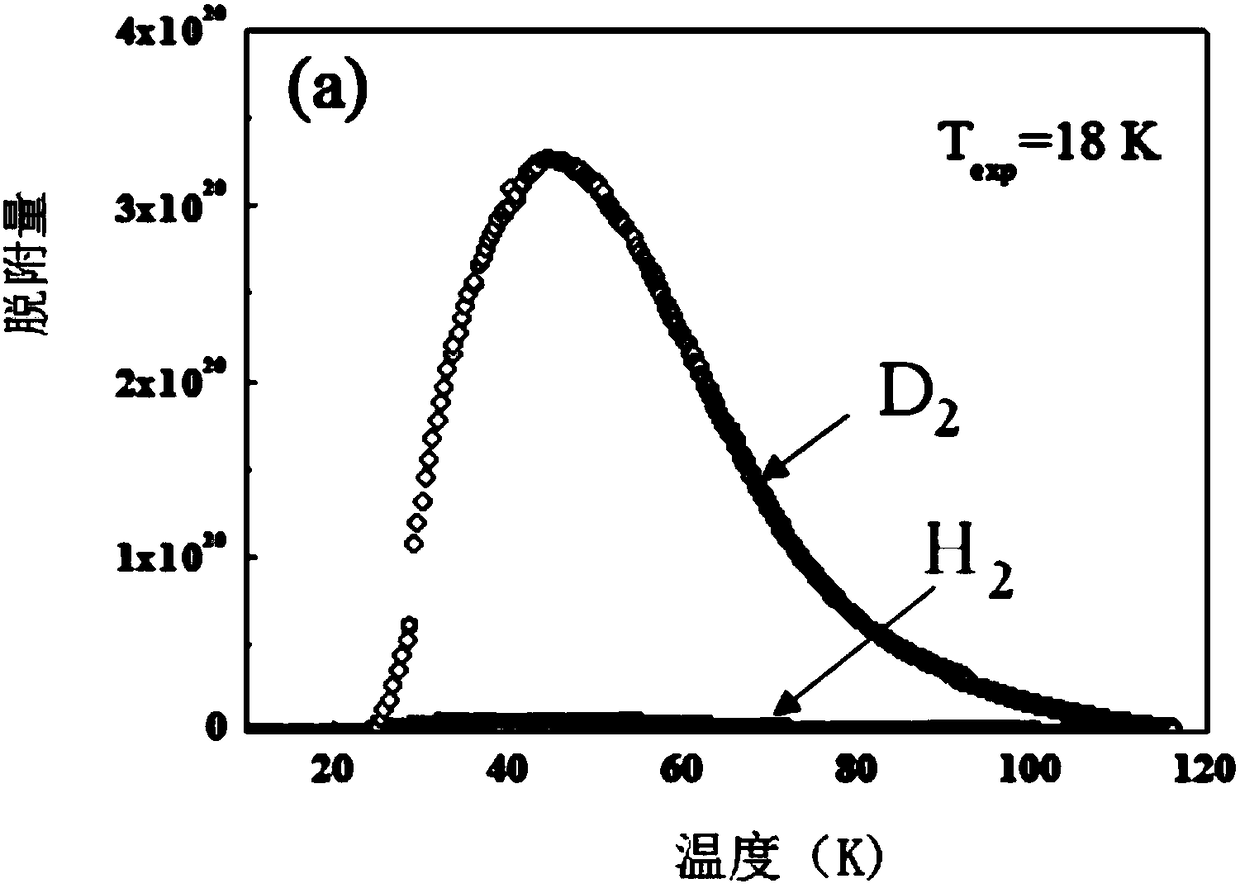
[0039] see Figure 1a , Figure 1b , Test of adsorption isotherms of H2, D2 and T2 pure components in SIFSIX-3-Zn.
[0040] Take 30-2000mg SIFSIX-3-Zn, put it into a sample tube that has been accurately weighed, and carry out vacuum degassing on the adsorption instrument. The degassing temperature is between 30-300 ° C, and the degassing time is between 1-12 hours. After the degassing is completed, the sample tube with the sample is accurately weighed again to obtain the sample mass, and at the same time, the sample tube is transferred to the workstation for testing. The test temperature is between 18K and 77K, and the purity of the gas obtained by the test is greater than 99.99%.
Embodiment 2
[0042] see Figure 2a , 2b , 2c, 2d, 2e, 2f, Intermittent adsorption separation performance test of H2, D2 and T2 mixture in SIFSIX-3-Zn,.
[0043]Take 3-5mg SIFSIX-3-Zn sample, put it in the sample cell, and perform vacuum degassing and activation between 30-300°C. After fully activated, cool the sample cell to the test temperature. After the system is balanced, inject the mixture of H2 / D2 / T2 with a specific ratio into the sample cell one or more times to make it reach a certain temperature between 1kPa and 100kPa. pressure point. After the adsorption equilibrium of the system, open the valve to quickly extract the unadsorbed gas mixture, and then close the valve. Cool down the system to a certain temperature between 18K-30K. After equilibrium, connect the sample cell to the mass spectrometer, and gradually raise the temperature. At the same time, use mass spectrometry to analyze the change of hydrogen isotope content over time. The adsorption selectivity can be calculate...
Embodiment 3
[0045] see image 3 , Performance test of fixed bed adsorption separation of H2, D2 and T2 mixture in SIFSIX-3-Zn.
[0046] Take 100-2000mg SIFSIX-3-Zn powder and put it into a packed bed column of a certain specification to ensure that the sample is packed compactly. At the same time, the samples in the packed bed were activated by vacuum degassing at 30–300 °C and purged with He for a certain period of time. After fully activated, cool the sample cell to the test temperature. After the system is balanced, pass a specific ratio of H2 / D2 / T2 mixture from one end of the fixed bed to the bed at a certain flow rate, and monitor the flow of gas from the other end with a mass spectrometer. Concentration changes until the effluent gas concentration remains stable and consistent with the inlet gas concentration. Due to the high selectivity of the material to hydrogen isotopes at 20K, the material can effectively separate D2 / H2 / T2 mixtures.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com