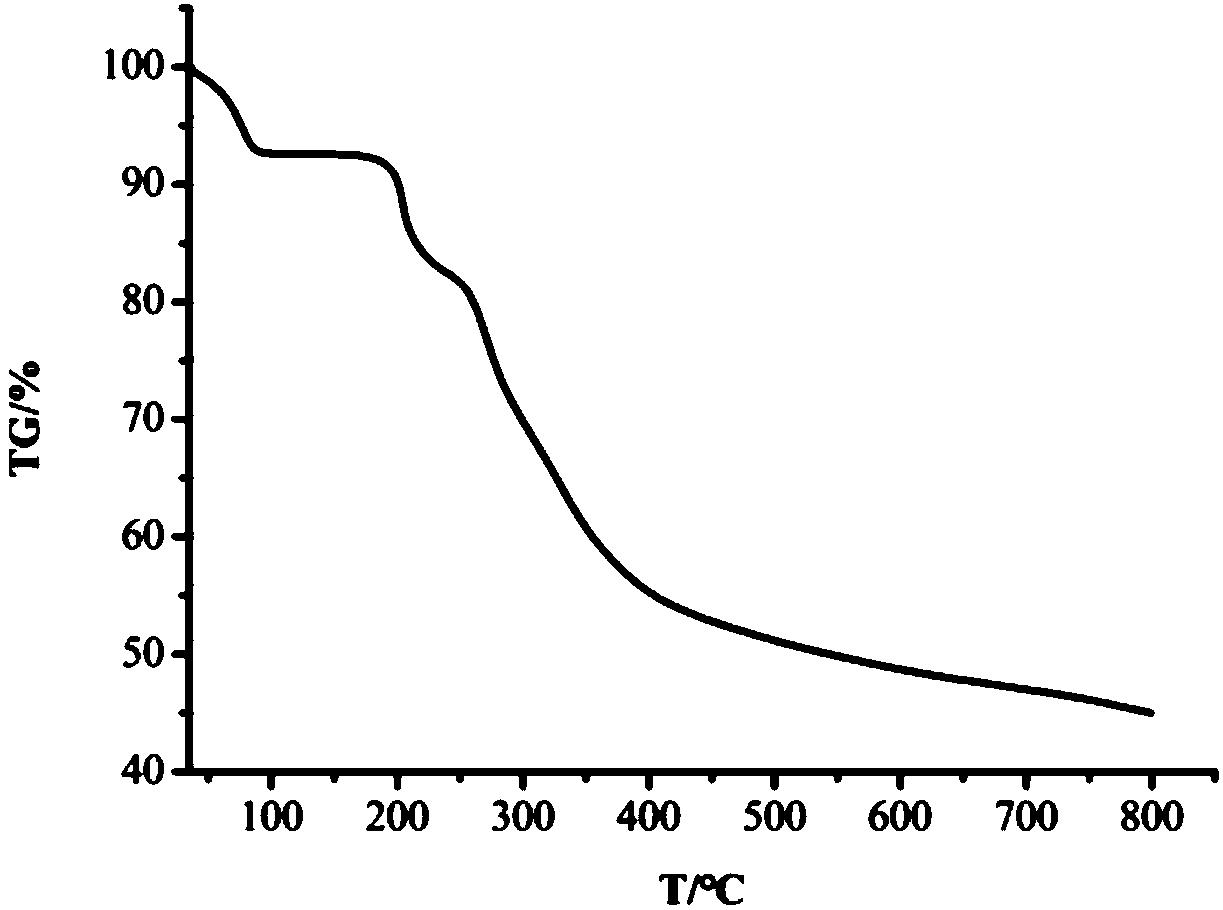
Copper complex based on biotin o-vanillin acylhydrazone derivative and synthesis method and application thereof
A technology of oxalyl hydrazone and copper complexes, which can be applied to copper organic compounds, 1/11 group organic compounds without C-metal bonds, drug combinations, etc. The effect of good proliferation inhibitory activity and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: The compound shown in formula (II) is the synthesis of biotin o-vanillin hydrazone ligand
[0033]
[0034]Weigh 2.58g of biotin hydrazide and 1.52g of o-vanillin into a container, add 150mL of ethanol, and reflux for 5h to obtain a yellow solid product with a yield of 78%.
[0035] NMR, mass spectrometry and infrared analysis are carried out to the product obtained in the present embodiment, and the obtained spectral data are respectively as follows:
[0036] 1 H NMR(400MHz,DMSO)δ11.57(s,0.62H),11.21(s,0.38H),10.89(s,0,62H),9.53(s,0.38H),8.34(s,0.38H), 8.27(s,0.62H),7.25–7.20(m,0.38H),7.09–7.05(m,0.62H),7.03–6.99(m,1H),6.87–6.77(m,1H),6.46(s, 1H),6.38(bs,1H),4.33–4.25(m,1H),4.18–4.10(m,1H),3.80(s,1.14H),3.79(s,1.86H),3.15–3.08(m, 1H),2.85–2.78(m,1H),2.63–2.55(m,1.86H),2.25–2.18(m,1.14H),1.73–1.27(m,6H).
[0037] ESI-MS m / z: [L-H] + , 392.15
[0038] IR(KBr,cm -1 ): 3791m, 3378s, 2928s, 1678s, 1452s, 1258s, 1149w, 1079w, 752m.
[0039] Therefore...
Embodiment 2
[0041] Embodiment 2: The compound shown in formula (II) is the synthesis of biotin o-vanillin hydrazone ligand
[0042] Example 1 was repeated, except that the reaction was carried out at 50° C. and the reaction time was 5 h. The yield was 67%.
[0043] The product obtained in this example was analyzed by NMR, mass spectrometry and infrared, and it was determined to be biotin-o-vanillyl hydrazone ligand.
Embodiment 3
[0044] Embodiment 3: copper complex [CuL(CH 3 OH)] NO 3 - ·H 2 Synthesis of O (hereinafter referred to as complex 1):
[0045] Weigh 0.1176g of biotin o-vanillin hydrazone ligand and dissolve it in 10mL of methanol, then add 0.0725g of copper nitrate trihydrate, and place it in a 70°C water bath with magnetic stirring for 2 hours to make the mixed solution uniform to obtain a dark green solution; After cooling the dark green solution, filter it, place the filtrate in a 50mL small beaker, seal 2-3 pinholes with a plastic wrap, and slowly evaporate the solvent at room temperature. After 8 days of evaporation, dark green flaky crystals are obtained. The crystals were washed three times with methanol, and then dried at 50° C. for 6 h to obtain dark green flaky crystals. Yield 34%.
[0046] The obtained dark green flaky crystals of the present embodiment are characterized:
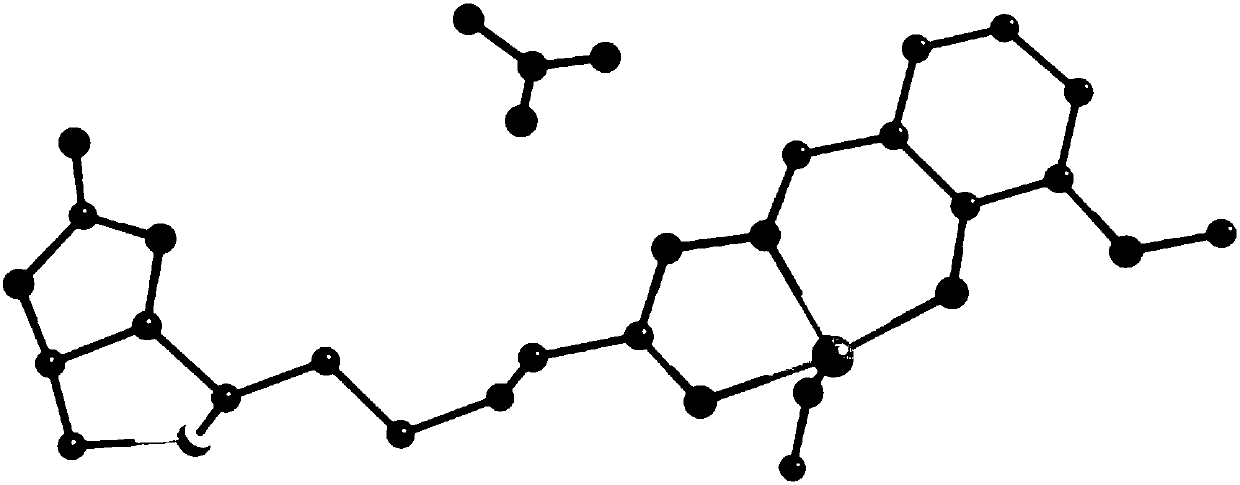
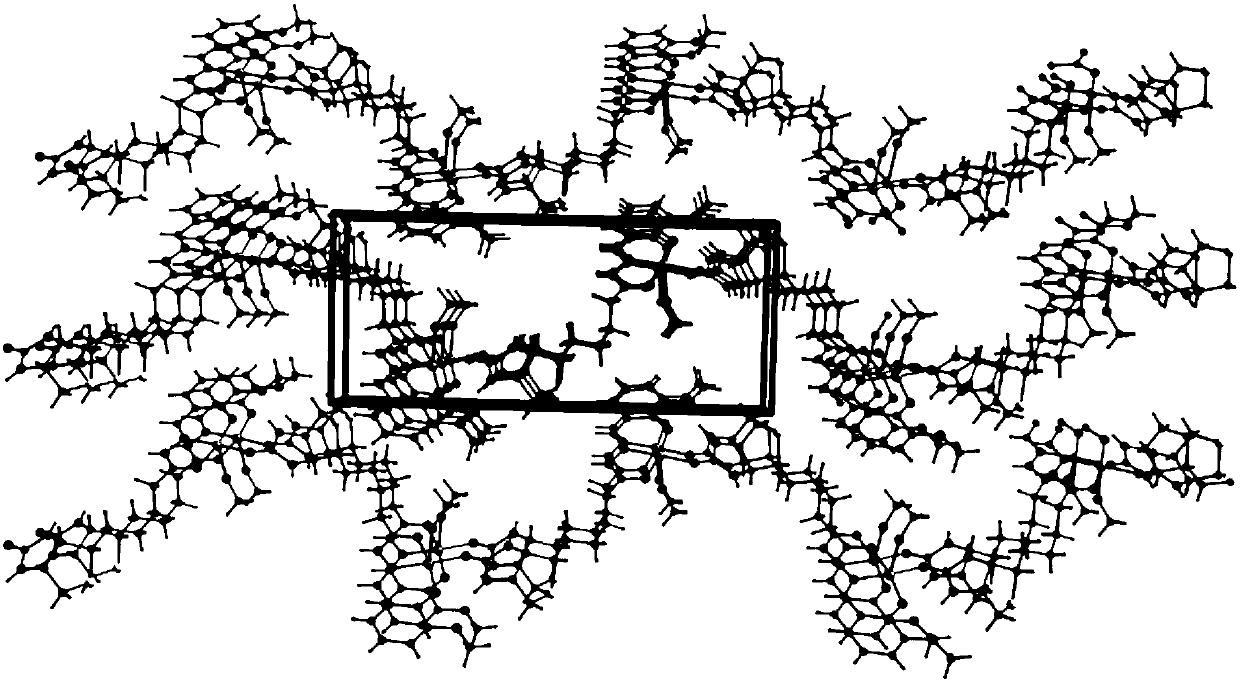
[0047] 1) Crystal structure analysis:
[0048] Single crystal X-ray analysis was performed on the dar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com