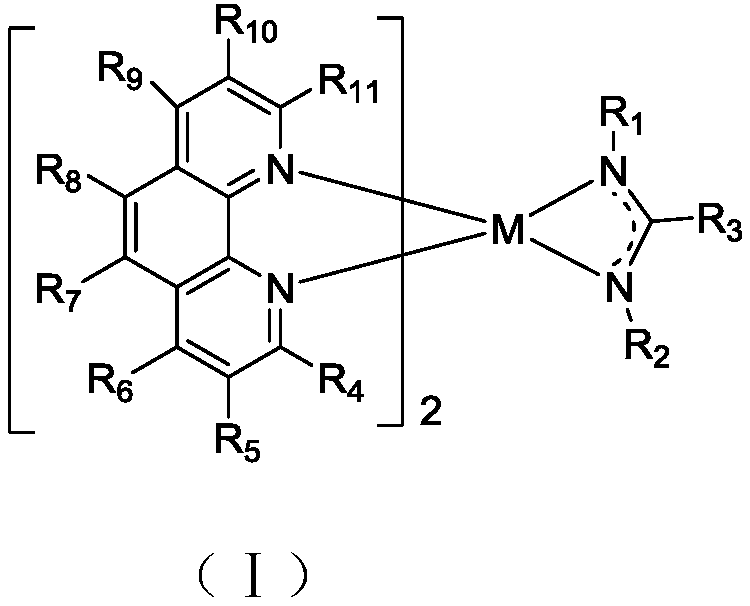
Phenanthroline-containing organic complex and organic light emitting device (OLED) thereof
An organic light-emitting device and organic complex technology, which are applied in the field of phenanthroline-containing organic complexes and organic light-emitting devices, can solve the problem that high-performance red phosphorescent materials need to be further developed, and meet the requirements of industrialization. The effect of demand, easy availability of raw materials, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
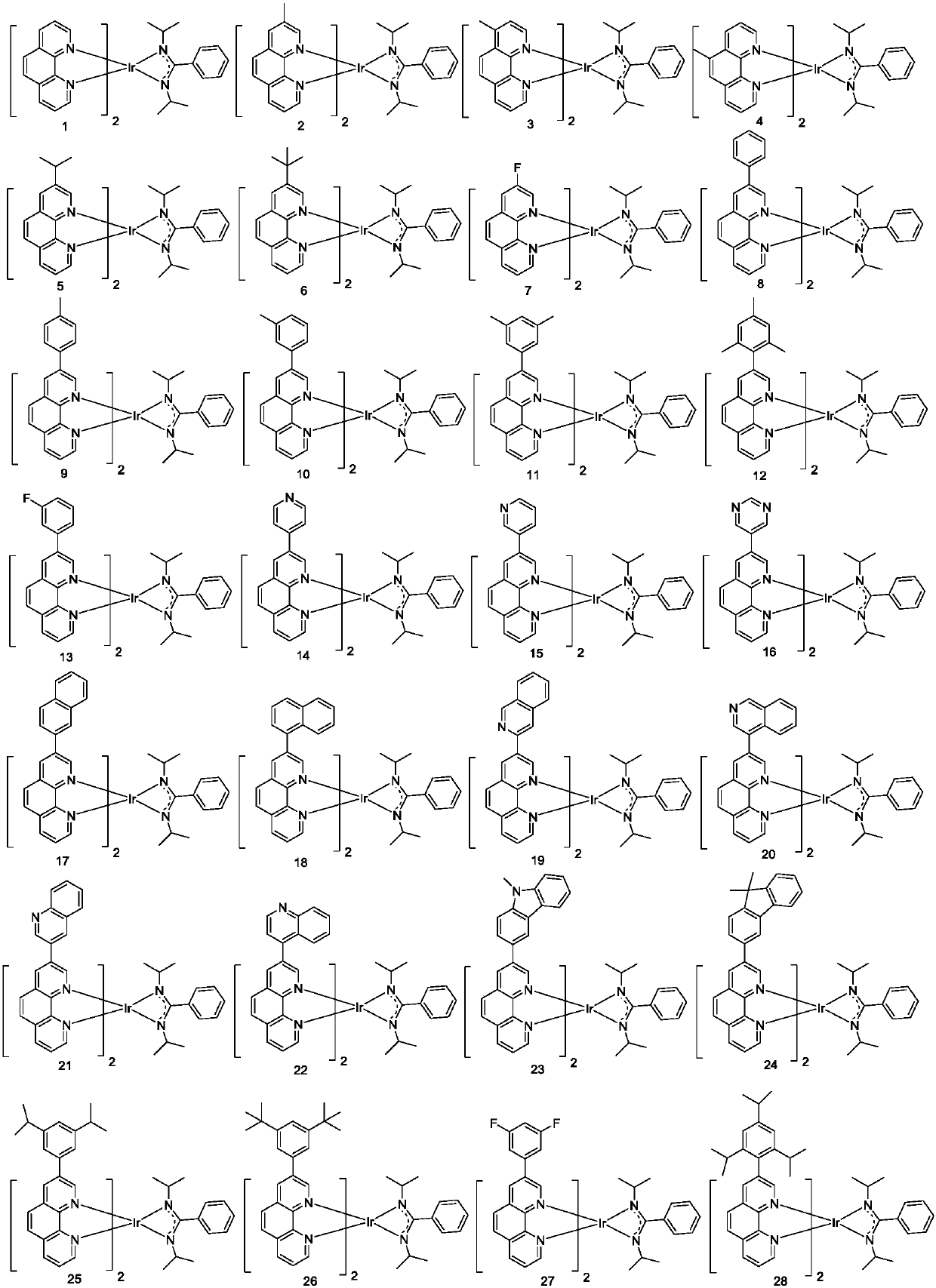
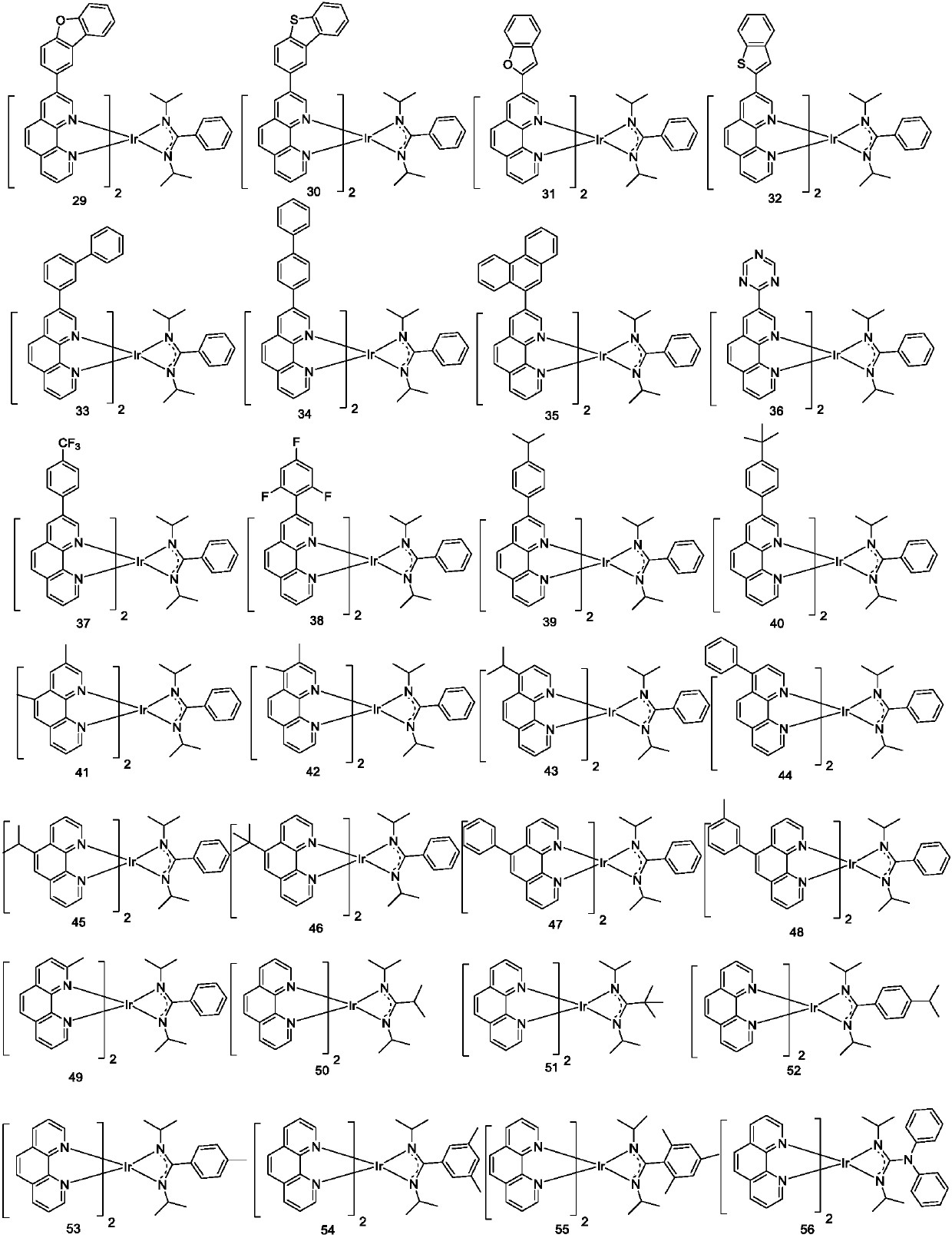
[0072] Embodiment 1: the preparation of compound 1
[0073] Preparation of Intermediate A1
[0074]
[0075] In a 1L three-necked flask, add iridium trichloride hydrate (14.1g, 40mmol) and compound 1,10 phenanthroline-e1 (35.1g, 170mmol), then add 300mL2-ethoxyethanol and 100mL water, and the mixture is Reflux overnight under nitrogen atmosphere. After the reaction, the temperature was lowered to room temperature, the precipitate was filtered, washed with methanol, and dried to obtain intermediate A1 (23.89 g, yield 90%).
[0076] Mass Spectrum m / z: 1327.29 (calculated: 1327.28). Theoretical element content (%)C 59 h 51 Cl 2 Ir 2 N 8 H, 3.87; Cl, 5.34; Ir, 28.96; N, 8.44 Measured element content (%): C, 53.39; H, 3.86; Cl, 5.34; The above results confirmed that the obtained product was the target product.
[0077] Preparation of Compound 1
[0078] Bromobenzene (3.1g, 20mmol) in n-hexane (100mL) was added to a 100mL four-neck flask, cooled to -80°C, and n-buty...
Embodiment 2
[0080] Embodiment 2: the preparation of compound 2
[0081]
[0082] Methylboronic acid (2.30g, 38.4mmol), c2 (7.95g, 30.7mmol) and K 2 CO 3 (8.48g, 61.4mmol) was dissolved in toluene (150ml) and water (30ml). The mixture was degassed by bubbling it with nitrogen for 30 minutes, and tetrakistriphenylphosphopalladium Pd (PPh 3 ) 4(1.78 g, 1.53 mmol). The mixture was then kept at 100°C overnight. After completing the reaction, the mixture was cooled to room temperature, extracted with toluene, and washed with brine and water. The organic solution was filtered and evaporated. The crude product was purified by column chromatography using a gradient mixture of ethyl acetate in heptane (20% to 65%). The white powder product was recrystallized three times from heptane to give e2 as colorless crystals. In a 1L three-necked flask, iridium trichloride hydrate (14.1g, 40mmol) and compound e2 (33.02g, 170mmol) were added, then 300mL of 2-ethoxyethanol and 100mL of water were ad...
Embodiment 3
[0087] Embodiment 3: the preparation of compound 8
[0088] The methyl bromide in Example 2 was replaced by equimolar bromobenzene, and the other steps were the same as in Example 2 to obtain the target compound 8 (5.27 g, 29%).
[0089]
[0090] Mass Spectrum m / z: 909.34 (calculated: 909.33). Theoretical element content (%)C 49 h 44 IrN 6 : C, 64.74; H, 4.88; Ir, 21.14; N, 9.24 Measured element content (%): C, 64.75; H, 4.88; Ir, 21.13; N, 9.24. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com