Method to separate enriched Z and E types of 3-chloro-2-(4-fluorophenyl)-1-(2-chlorophenyl)propylene
A technology of separation and enrichment, fluorophenyl, applied in organic chemistry methods, halogenated hydrocarbon disproportionation separation/purification, organic chemistry, etc., can solve the problems of inability to produce high purity, unstable performance, low technical level, etc. Stability of product quality and yield, shortened production and processing cycles, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
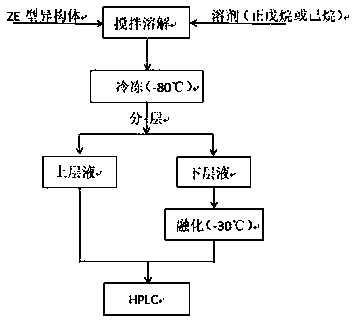
[0025] 1. Rotate the mixed solution rich in Z-type and E-type 3-chloro-2-(4-fluorophenyl)-1-(2-chlorophenyl)propene at 50°C and 80°C respectively to remove methanol and Toluene;
[0026] 2. Take four clean and dry beakers (number: hexane 1, hexane 2, hexane 3, hexane 4), add 15mL of hexane into each beaker, then add 2mL, 5mL, 10mL and 15mL of the above-mentioned rotary evaporation liquid was obtained to obtain the first solution;
[0027] 3. Freeze the solution in a -80°C refrigerator;
[0028] 4. After about 4-6 hours, stratification occurs, and the upper layer is a colorless transparent liquid. The upper liquid is separated at low temperature to obtain the second solution, which is sampled for HPLC. The numbers are hexane 1-up, hexane 2-up, hexane Alkane 3-on, hexane 4-on;
[0029] 5. The lower layer is a dark brown solid. Place it in a refrigerator at -30°C. After about 6 minutes, it will melt, and the third solution will be obtained. Samples will be tested for HPLC. -X...
Embodiment 2
[0035] 1. Rotate the mixed solution rich in Z-type and E-type 3-chloro-2-(4-fluorophenyl)-1-(2-chlorophenyl)propene at 50°C and 80°C respectively to remove methanol and Toluene;
[0036] 2. Take four clean and dry beakers (number: n-pentane 1, n-pentane 2, n-pentane 3, n-pentane 4), add 15mL of n-pentane to each beaker, and then add 2mL dropwise , 5mL, 10mL and 15mL of the above rotary evaporation liquid to obtain the first solution;
[0037] 3. Freeze the solution in a -80°C refrigerator;
[0038] 4. After about 4-6 hours, stratification occurs. The upper layer is a colorless transparent liquid. The upper liquid is separated at low temperature to obtain the second solution, which is sampled for HPLC. The numbers are n-pentane 1-up and n-pentane 2-up respectively. , n-pentane 3-up, n-pentane 4-up;
[0039] 5. The lower layer is a dark brown solid, placed in a refrigerator at -30°C, it will melt after about 2 minutes, and the third solution is obtained, and samples are taken...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com