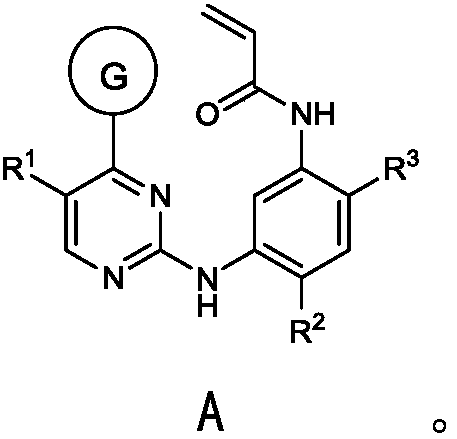
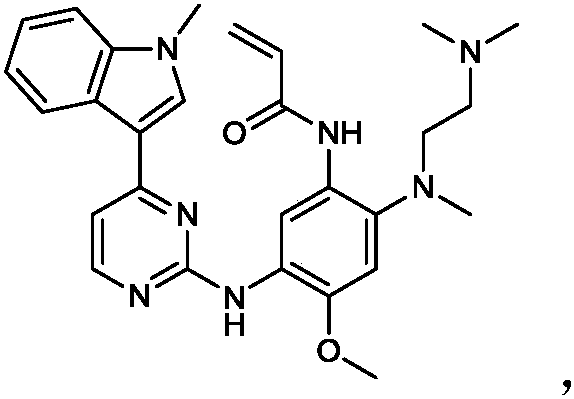
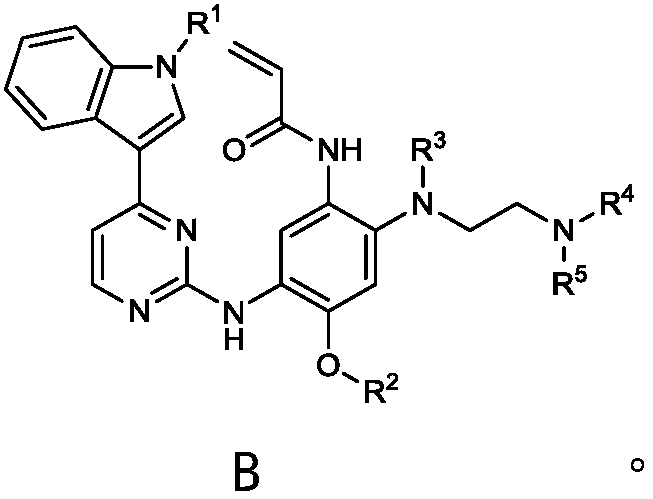
Deuterated osimertinib derivatives and application thereof
A compound and solvate technology, applied in the field of medicine, can solve the problems of tumor patients losing efficacy, achieve the effect of reducing inhibitory activity, increasing inhibitory activity, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Target compound 1, the structural formula is:
[0043]
[0044] The synthetic route is:
[0045]
[0046] Concrete synthetic steps are as follows:
[0047] 1) Synthesis of compound TRN158-a: under nitrogen protection, add 18 grams of 5-bromo-2,4-dichloropyrimidine and 180 milliliters of anhydrous tetrahydrofuran to a 1-liter three-necked flask, drop to -60 °C, The tetrahydrofuran solution of isopropylmagnesium chloride was 393 ml for 40 minutes; then the temperature was raised to -30°C for 1 hour, and 20 ml of deuterium water was added dropwise at -30°C for 20 minutes, and then slowly raised to room temperature for 1 hour; After completion, add 1 liter of ethyl acetate to the reaction solution and stir for 10 minutes, filter, and spin the filtrate to dryness; the obtained crude product is subjected to silica gel column chromatography to obtain 3.07 g of white solid; MS+1: 151.
[0048] 2) Synthesis of compound TRN158-b: under the protection of nitrogen, add 4 gr...
Embodiment 2
[0055] Target compound 2, the structural formula is as follows:
[0056]
[0057] The synthetic route is as follows:
[0058]
[0059] Synthesis of compound TRN15802-2:
[0060] Compound TRN15801-1 (500mg, 2.02mmol) was added to the reaction flask, then 10 ml of isopropanol was added, TRN158-d (416mg, 2.2mmol) and p-toluenesulfonic acid monohydrate (475mg, 2.5mmol) were added, Then, under the protection of argon, reflux reaction was added overnight. The reaction solution was lowered to room temperature, then concentrated to remove part of the solvent, then placed in an ice bath, left to stand for crystallization, filtered, the filter cake was washed twice with acetonitrile, and dried to obtain 524 mg of the product. MS+1: 401.3.
[0061] Synthesis of compound TRN15802-3:
[0062] Compound TRN15802-2 (520mg, 1.3mmol), N,N,N'-trimethylethylenediamine (265mg, 2.6mmol) and potassium carbonate (359mg, 2.6mmol) were added to the reaction flask, and then 10 Milliliter of N...
Embodiment 3
[0070] Target compound 3, the structural formula is as follows:
[0071]
[0072] The synthetic route is as follows:
[0073]
[0074] The intermediate TRN158-e was purchased from Bailingwei Reagent Company.
[0075] The synthetic route of intermediate TRN158-f is as follows:
[0076]
[0077] Synthesis of compound 3:
[0078] Compound 1 (5 g, 71 mmol) was added into a three-necked flask, and then 100 ml of ethanol, triethylamine (7.2 g, 71 mmol), and benzaldehyde (7.5 g, 71 mmol) were added at 0°C. Tetraisopropyl titanate (21.6 g, 76 mmol) was added dropwise at 0°C, and after the addition was completed, it was slowly raised to room temperature overnight. The next day, the reaction solution was lowered to 0°C, and sodium borohydride (2.8g, 74mmol) was added in batches within 2 hours. After the addition, the reaction was continued at 0°C for 4 hours, and saturated ammonium chloride aqueous solution was added to quench The reaction was quenched, filtered, and the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com