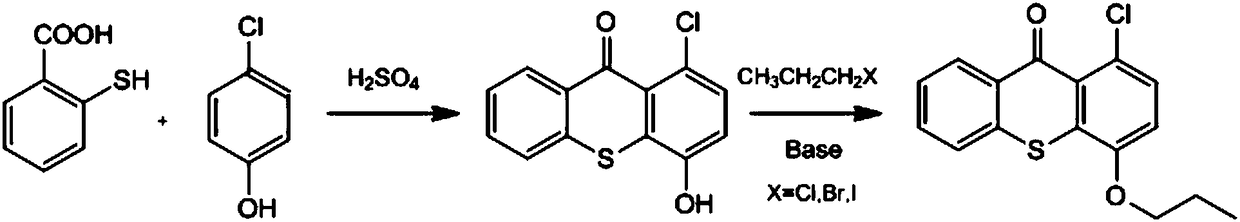
Preparation method of photoinitiator 1-chloro-4-propoxythioxanthone
A technology of propoxythioxanthone and photoinitiator, applied in organic chemistry and other directions, can solve problems such as low yield and dark product color, and achieve the effects of simple post-processing, reduced raw material cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Condensation ring reaction: Add 48 grams of p-chlorophenol and 40 grams of 2-mercaptobenzoic acid into a 1000ml four-necked bottle filled with 240 grams of 88-90% concentrated sulfuric acid, heat up to 60°C and react for 6-10 hours After the reaction, the reaction solution was cooled to room temperature, poured into 300 grams of ice water and 200 grams of methyl ethyl ketone, mixed and stirred, left to stand to obtain a layered solution, and the upper aqueous layer was removed to obtain the lower organic layer.
[0026] (2) Etherification reaction: Add 27 grams of potassium carbonate and 38 grams of bromopropane to the above organic layer, heat up to 80° C. and react for more than 5 hours under stirring, and cool down to 40° C. after HPLC detection until the intermediate reaction is complete.
[0027] (3) 260 g of water was added to the above reaction system for washing, and the layers were separated after standing, and the obtained organic layer was subjected to des...
Embodiment 2
[0030] (1) Condensation ring reaction: Add 20 grams of p-chlorophenol and 20 grams of 2-mercaptobenzoic acid into a 1000ml four-necked bottle filled with 200 grams of 88-90% concentrated sulfuric acid, heat up to 70°C and react for 6-10 hours After the reaction, the reaction solution was cooled to room temperature, poured into 300 grams of ice water and 140 grams of methyl ethyl ketone, mixed and stirred, left to stand to obtain a layered solution, and the upper aqueous layer was removed to obtain the lower organic layer.
[0031] (2) Etherification reaction: Add 20 grams of potassium carbonate and 24 grams of bromopropane to the above organic layer, heat up to 80° C. and react for more than 5 hours under stirring, and cool down to 40° C. after HPLC detection until the intermediate reaction is complete.
[0032] (3) Add 250 g of water to the above reaction system for washing, let stand to separate the layers, and carry out desolvation of the obtained organic layer under reduced...
Embodiment 3
[0035] (1) Condensation ring reaction: 35 grams of p-chlorophenol and 30 grams of 2-mercaptobenzoic acid are added to a 1000ml four-necked bottle of 400 grams of 88-90% concentrated sulfuric acid, and the temperature is raised to 70°C for 6-10 hours. After the reaction, the reaction solution was cooled to room temperature, poured into 400 g of ice water and 300 g of methyl ethyl ketone, mixed and stirred, and allowed to stand to obtain a layered solution. The upper aqueous layer was removed to obtain the lower organic layer.
[0036] (2) Etherification reaction: Add 29 grams of potassium carbonate and 33 grams of bromopropane to the above organic layer, heat up to 80° C. and react for more than 5 hours under stirring. After the intermediate reaction is completely detected by HPLC, the temperature is lowered to 40° C.
[0037] (3) 340 g of water was added to the above reaction system for washing, and the layers were separated after standing, and the obtained organic layer was su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com