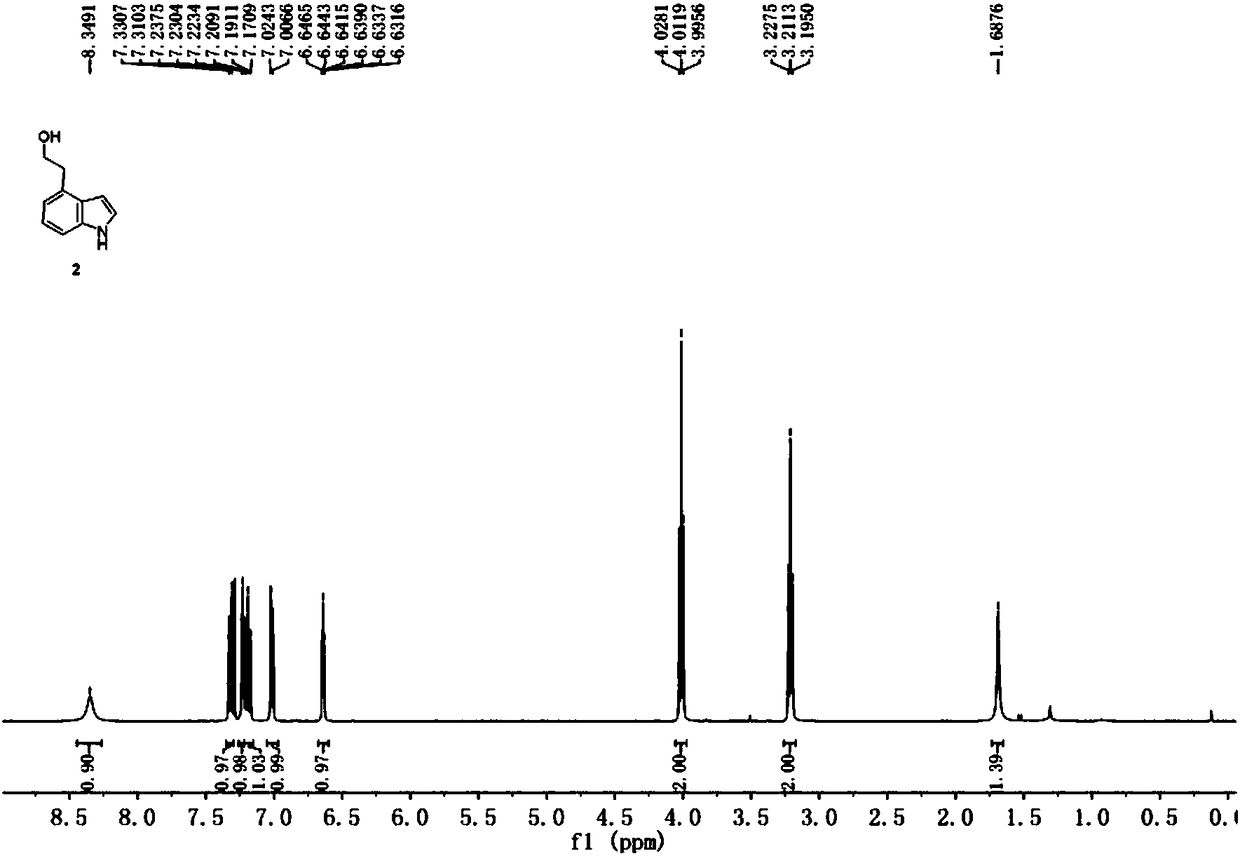
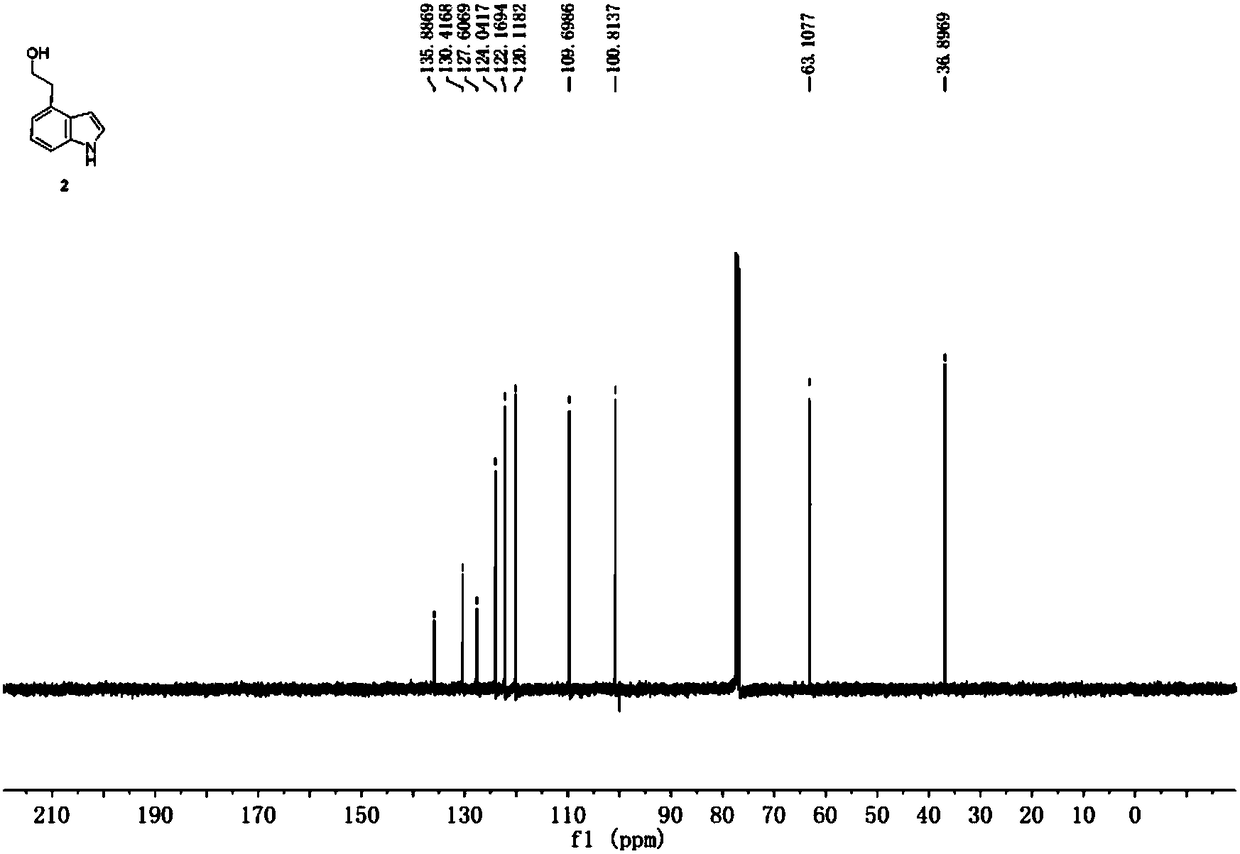
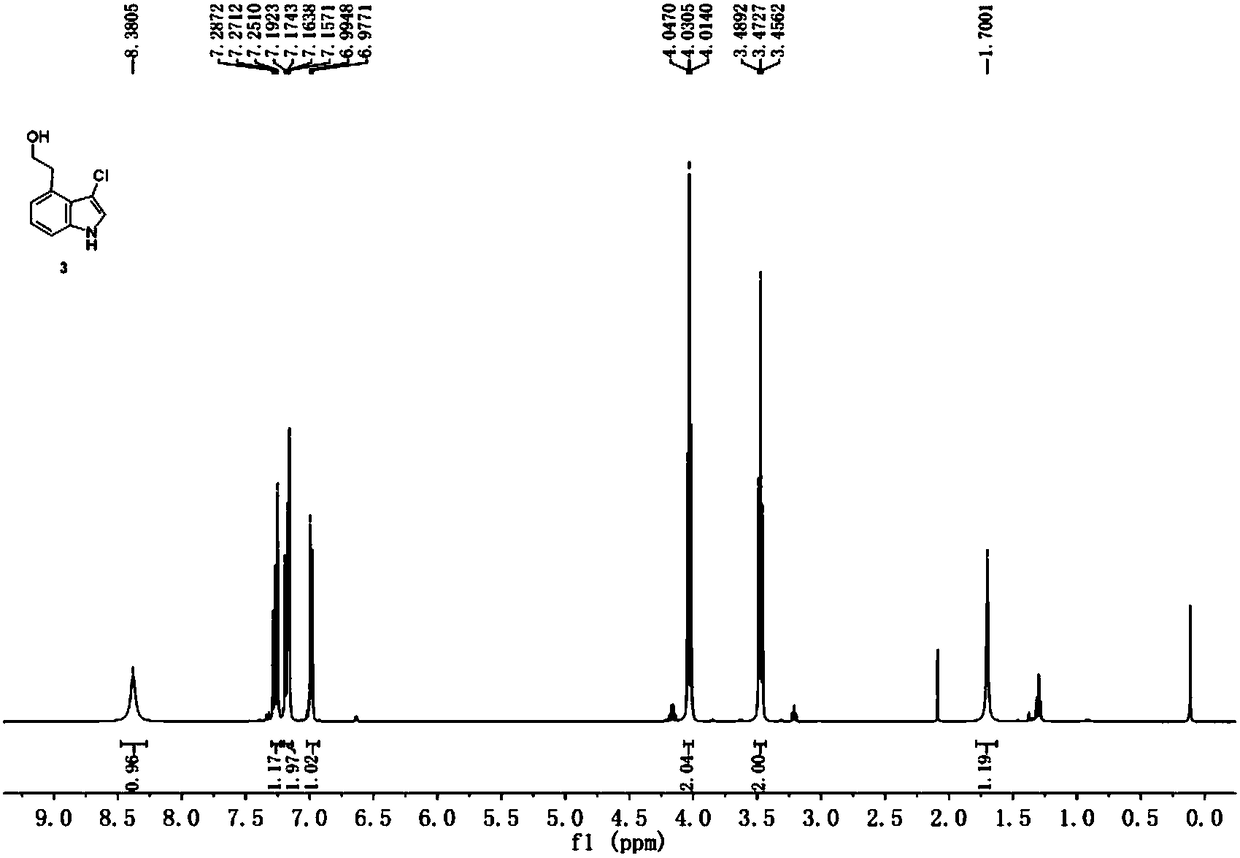
Method for preparing ropinirole hydrochloride
A technology of ropinirole hydrochloride and ethanol, which is applied in the field of preparation of ropinirole hydrochloride, can solve the problems of complex reaction and low yield, and achieve the effects of simple preparation process, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: synthetic compound 4
[0074]1. Compound 3 was divided into 15 groups, each group 39 mg. Using a dry round-bottomed flask as a reaction vessel, dissolve compound 3 in each group of solvents (subject to complete dissolution) at a temperature of -40°C to 80°C, slowly add 1.1 equivalents of component 1, and then slowly add 1.1 Equivalent of component 2, continue to stir the reaction for no more than 30 seconds. TLC monitors that the reaction is complete, adding water with a volume 5 to 10 times the volume of the solvent, extracting 3 times with ethyl acetate, combining the organic phases and then extracting with saturated brine, drying with anhydrous sodium sulfate, concentrating under reduced pressure to obtain a crude product, and passing through the column Chromatographic purification afforded compound 4.
[0075] The specific parameters and effects of each group are shown in Table 1.
[0076] Each concrete parameter and yield of synthetic compound 4 of...
Embodiment 2
[0080] Embodiment 2: synthetic compound 5
[0081] 1. Divide compound 4 into 6 groups, each 42 mg, using a dry two-necked bottle as a reaction vessel, under hydrogen ambient conditions, dissolve each group of compound 4 in a solvent (subject to complete dissolution), add at room temperature Pd / C of different quality, continue stirring reaction for about 2 hours. The completion of the reaction was monitored by TLC, and the solid was filtered off with diatomaceous earth. The filtrate was concentrated under reduced pressure to obtain a crude product, which was purified by column chromatography to obtain compound 5.
[0082] The specific selection of solvent, the amount of Pd / C and the effect are shown in Table 2, wherein the amount of Pd / C refers to the ratio (%) of the mass of Pd / C to the mass of compound 4.
[0083] Table 2 The specific parameters and yields of synthetic compound 5
[0084] group
Solvent (2mL)
Pd / C dosage
Yield of Compound 5
group ...
Embodiment 3
[0087] Embodiment 3: synthetic compound 6
[0088] 1. Divide compound 5 into 4 groups, 35 mg in each group, use a dry round bottom flask as a reaction vessel, dissolve each group of compound 5 in dichloromethane (group 1), chloroform (group 2), 1,2-dichloroethane (group 3), pyridine (group 4) (subject to complete dissolution), add 1.2 equivalents of pyridine at room temperature, then add 1.2 equivalents of p-toluenesulfonyl chloride, continue to stir and react for about 2 hours. TLC monitored the reaction to be complete, adding 1N hydrochloric acid solution to quench the reaction, using ethyl acetate to extract 3 times, combining the organic phases and then extracting with saturated brine, drying with anhydrous sodium sulfate, concentrating under reduced pressure to obtain a crude product, and then purifying by column chromatography Compound 6 was obtained.
[0089] 2. The effects of each group are shown in Table 3.
[0090] Table 3 shows the effect of synthesizing compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com