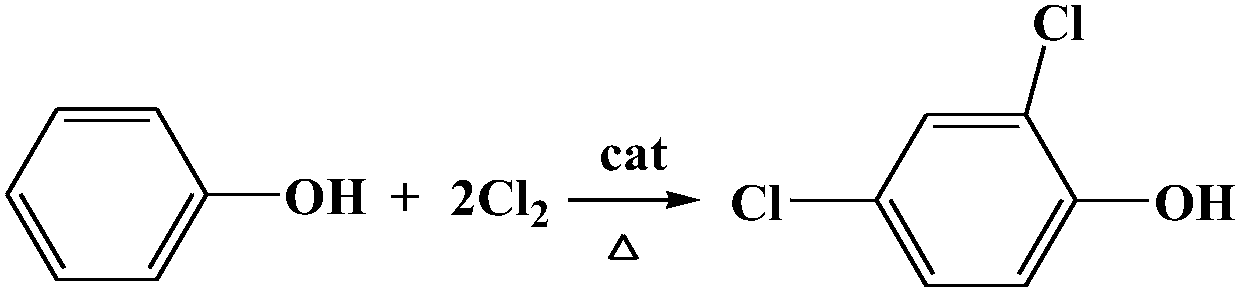
Production method and production system for 2,4-dichlorophenol
A production system and production method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high environmental protection costs, difficulty in controlling sulfur dioxide reuse, and poor product appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] supply figure 1 The production system shown; the aspect ratio of the fixed bed reactor is 10, the flow velocity of phenol is 0.2m / s; the flow velocity of sulfuryl chloride is 0.1m / s.
[0056] Dissolve 1 kg of ferric chloride in 100 kg of methanol, mix with 50 kg of polyacrylic acid ion exchange resin, reflux for 5 hours, remove methanol and then dry in vacuum to obtain a supported metal salt catalyst.
[0057] The loaded metal salt catalyst is filled into the tubes of the ceramic medium fixed-bed reactor, and sulfuryl chloride distributors are arranged between the tubes.
[0058] Mix 100kg phenol with 0.01kg dipropyl sulfide to obtain a mixed solution;
[0059] Pass the mixed liquid and sulfuryl chloride into the fixed bed reactor according to the equimolar ratio of phenol and sulfuryl chloride to carry out the chlorination reaction, and the temperature of the fixed bed reactor is controlled at 80°C through the shell-side heat exchanger.
[0060] The chlorination reac...
Embodiment 2
[0065] Continue to use the production system in Example 1.
[0066] Mix 100kg of phenol with 0.1kg of phenylene sulfide to obtain a mixed solution;
[0067] Pass the mixed liquid and sulfuryl chloride into the fixed bed reactor according to the equimolar ratio of phenol and sulfuryl chloride to carry out the chlorination reaction, and the temperature of the fixed bed reactor is controlled at 60°C through the shell-side heat exchanger.
[0068] The chlorination reaction solution continuously enters the deacidification tower to realize continuous deacidification. The tail gas of deacidification is a mixture of hydrogen chloride and sulfur dioxide, which is continuously passed into the sulfuryl chloride reactor, and continuously reacts with chlorine to generate sulfuryl chloride, which is then used for phenol chlorination and deacidification. The temperature is 80°C.
[0069] The deacidification liquid qualified for deacidification is continuously transferred to the rectificatio...
Embodiment 3
[0071] Get 0.08kg of phenylene sulfide recovered in Example 2, add it into 100kg of phenol, and mix thoroughly. Then the mixed solution and sulfuryl chloride were passed into the fixed-bed reactor in Example 2 according to the molar ratio of phenol and sulfuryl chloride to carry out the chlorination reaction, and the temperature of the fixed-bed reactor was controlled at 60° C. through a shell-side heat exchanger. Pass the mixed liquid and sulfuryl chloride into the fixed bed reactor according to the equimolar ratio of phenol and sulfuryl chloride to carry out the chlorination reaction, and the temperature of the fixed bed reactor is controlled at 120°C through the shell-side heat exchanger. The deacidification qualified deacidification liquid is continuously transferred to the rectification tower, and the phenol and ether catalysts are separated to obtain 0.9 kg of recovered phenylene sulfide catalyst and 173.0 kg of 2,4-dichlorophenol, with a content of 99.6% and a yield of 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com