A pharmaceutical composition for treating epileptic convulsions, infantile convulsions, and hemifacial spasm and its preparation method
A technology for infantile convulsions and hemifacial spasm, applied to the pharmaceutical composition and its preparation for the treatment of epileptic convulsions and hemifacial spasm, the field of convulsions in children, the drug is epilepsy capsules, which can solve frequent shutdowns for cleaning and moisture absorption of capsule contents , load difference and other issues, to achieve the effect of saving production cost and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 medicinal material extract
[0029] The medicinal material extract of this embodiment is prepared through the following steps:
[0030] 1. Take 1400g of Astragalus (here, 1 weight part = 10g, the same below), 1400g of Codonopsis pilosula, 1400g of Salvia miltiorrhiza, 700g of Bupleurum, 1050g of Suanzaoren, 700g of Polygala, 1050g of Tianma, 1050g of Uncaria, 700g of Shichangpu, and 700g of Dannanxing , Angelica 1400g, silkworm 1050g, Liushenqu 700g, turmeric 700g, licorice 700g, white aconite 350g.
[0031] 2. Crush silkworm and Liushenqu into fine powder, pass through 80-100 mesh sieve, and set aside;
[0032] 3. Take the other fourteen herbs such as astragalus and add water to decoct three times. Add 10 times the amount of water for the first time and decoct for 2 hours, add 8 times the amount of water for the second time and decoct for 1.5 hours, add 6 times the amount of water for the third time and decoct for 1 hours, combined the...
Embodiment 2
[0033] The screening test of adjuvant kind in the pharmaceutical composition described in embodiment 2
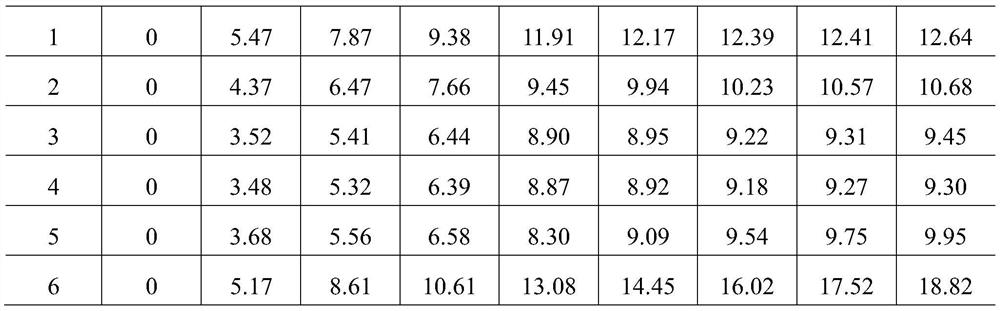
[0034] Get the medicine extract 50g that embodiment 1 prepares, be divided into 6 parts on average, add respectively the soluble starch of identical weight (5g), microcrystalline cellulose, calcium carbonate, calcium hydrogen phosphate, lactose, former drug powder (bomby silkworm and Liu Shenqu Mix according to the ratio of 10.5:7, grind, sieve), respectively recorded as 1# (soluble starch), 2# (microcrystalline cellulose), 3# (calcium carbonate), 4# (calcium hydrogen phosphate), 5# (Lactose), 6# (original drug powder), mix well, weigh 2g each precisely, spread them in a flat weighing bottle that has been dried to constant weight, and then place them in a glass desiccator filled with a supersaturated solution of sodium chloride at the bottom , Precisely weighed at 0, 4, 8, 12, 24, 36, 48, 72, and 96 hours; each auxiliary material was made in parallel in 3 copies, and the mo...
Embodiment 3
[0040] Example 3 Excipient dosage ratio screening test
[0041] Get 8 parts (40g each) of the medicinal extract prepared in Example 1, add calcium carbonate and / or calcium hydrogen phosphate in different weight ratios as shown in Table 2, mix uniformly, accurately weigh 2g respectively and spread it on a dry to constant In a heavy flat weighing bottle, place it in a glass desiccator filled with supersaturated solution of sodium chloride at the bottom for 24 hours, take it out, and weigh it accurately again; make 3 parts of each proportion of auxiliary materials in parallel, and calculate the percentage of moisture absorption % according to formula A , take the mean value. The results are shown in Table 2.
[0042] Table 2 Excipient ratio / dosage screening test results
[0043]
[0044] a : the ratio refers to the percentage by weight of the drug extract.
[0045] The results in Table 2 show that:
[0046] 1) The moisture absorption rate of the composition is significant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com