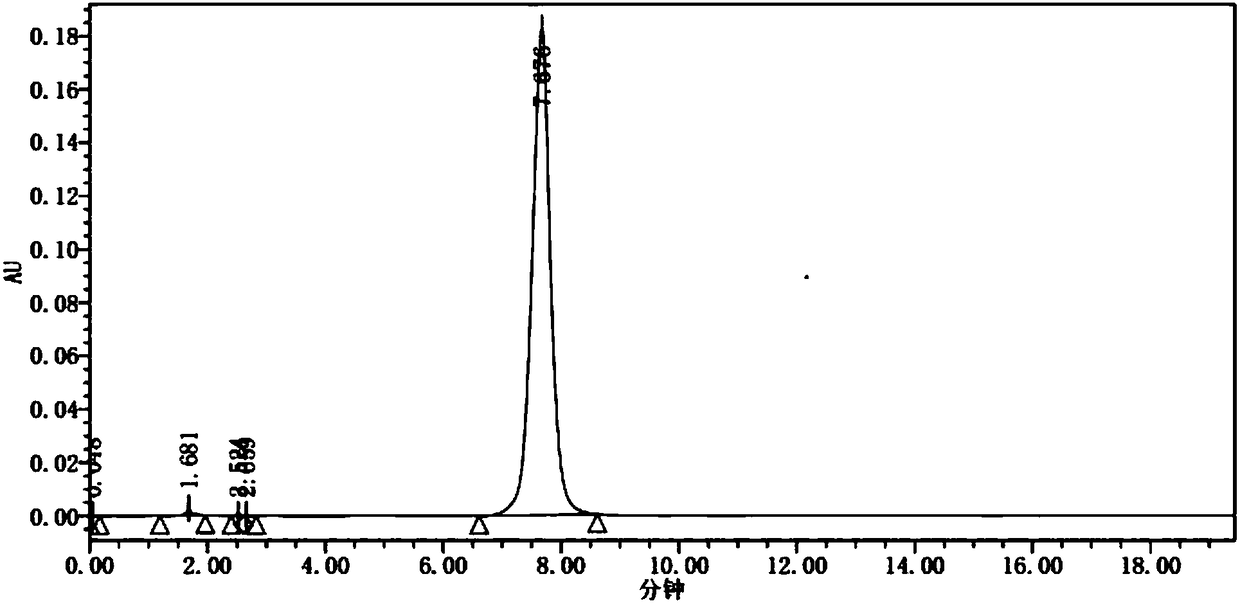
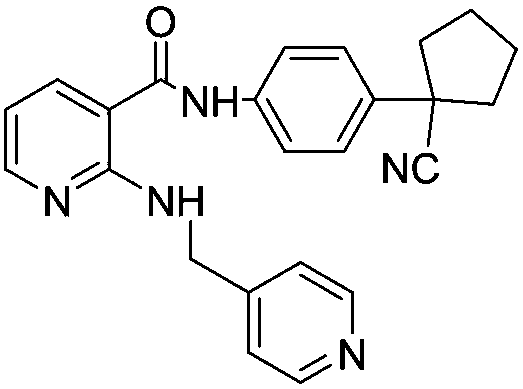
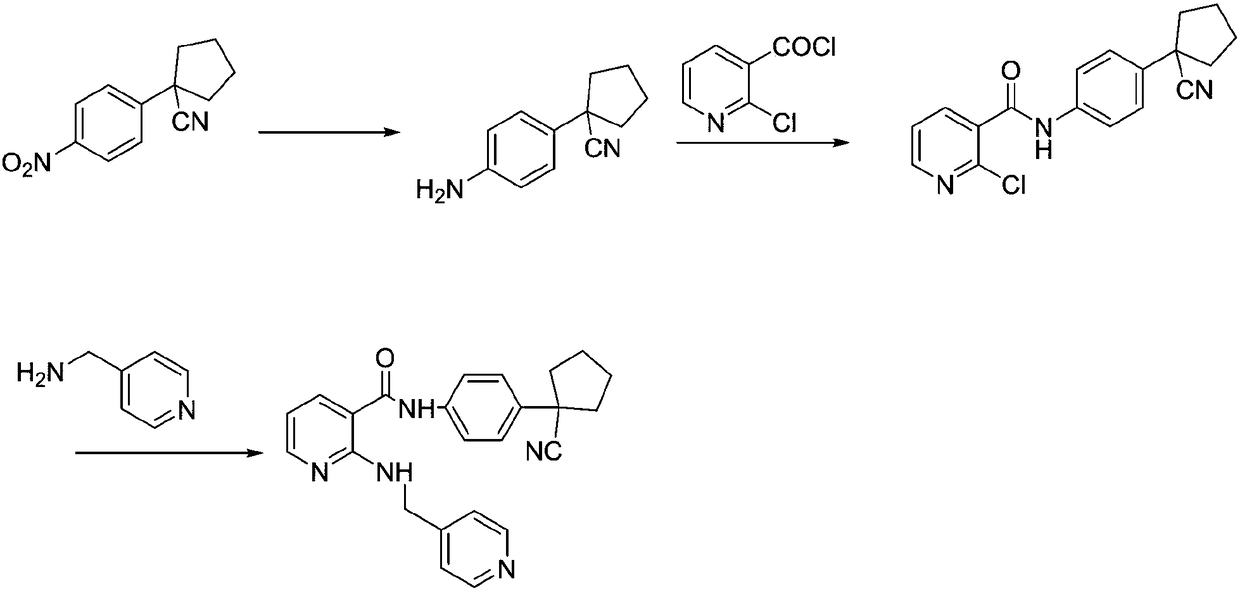
Preparation method of apatinib
A technology of apatinib and compounds, applied in the field of preparation of apatinib, can solve problems such as increased cost, harsh conditions, and increased difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: the preparation of 2-(4-aminophenyl) acetonitrile (compound 2)
[0092] Compound 1 (10g, 61.67mmol) was dissolved in methanol (50ml), added 10% Pd / C (1.0g), and washed with H 2 Replacement reaction system four times, in H 2 Stir overnight at room temperature under ambient (0.5 MPa). TLC analysis showed that the reaction was complete. The reaction liquid was filtered through diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain 8.08 g of compound 2 with a yield of 99.1%.
Embodiment 2
[0093] Embodiment 2: the preparation of 2-(4-aminophenyl) acetonitrile (compound 2)
[0094] Compound 1 (10g, 61.67mmol) was dissolved in methanol (50ml), added 5% Pd / C (1.2g), and washed with H 2 Replacement reaction system four times, in H 2 Stir overnight at room temperature under ambient (1 MPa). TLC analysis showed that the reaction was complete. The reaction liquid was filtered through celite, and the filtrate was concentrated under reduced pressure to obtain 7.99 g of compound 2 with a yield of 98.1%.
Embodiment 3
[0095] Example 3: Preparation of N-(4-(aminomethyl)phenyl)-2-nitronicotinamide (compound 4)
[0096] Compound 3 (16.8 g, 0.1 mol) was dissolved in acetonitrile (100 ml), carbonyldiimidazole (19.46 g, 0.12 mol) was added, and stirred at room temperature for 2 hours. Compound 2 (17.18 g, 0.13 mol) was added, the temperature was raised to 85° C., and the temperature was kept for 6 hours. After the reaction, the acetonitrile was distilled off to obtain an oil, and pure water (80ml) was added thereto, stirred with a glass rod to precipitate a solid, filtered, rinsed with water, and dried to obtain 22.92g of compound 4 with a yield of 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com