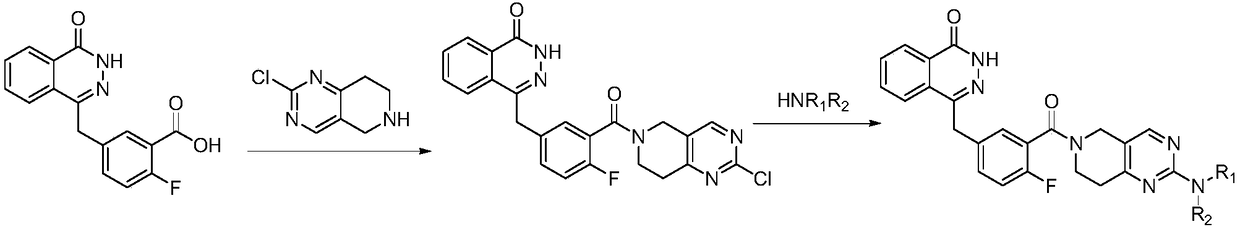
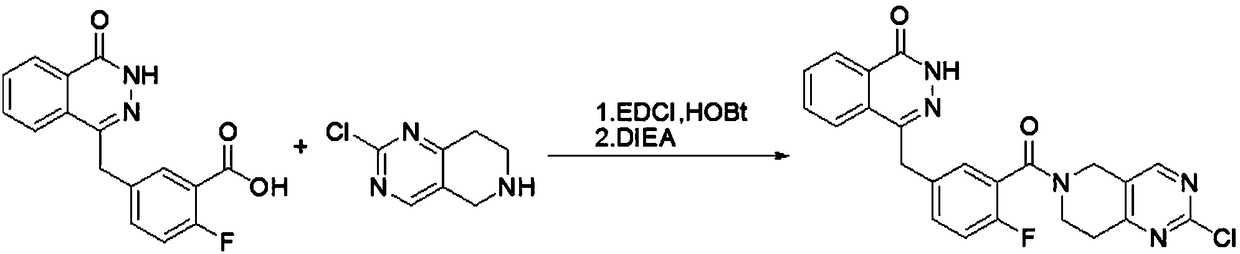
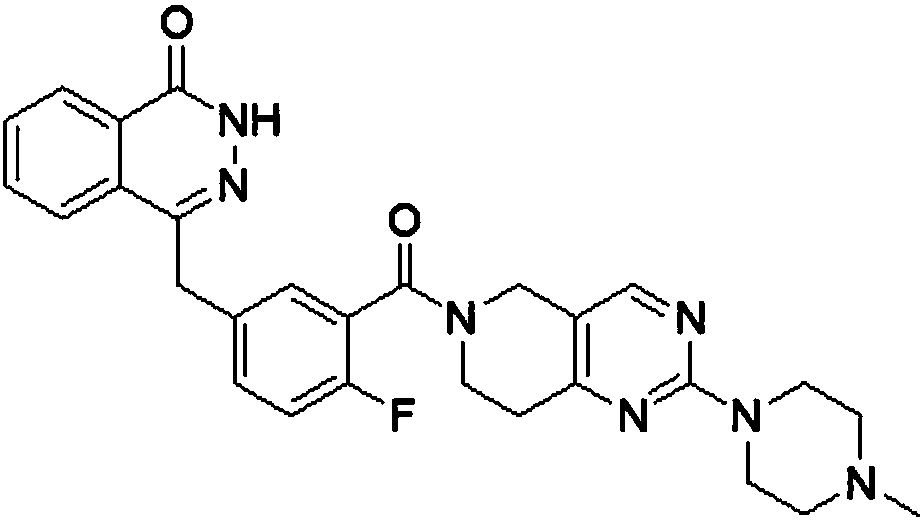
Amino-substituted tetrahydropyridinopyrimidine compound or usable salt thereof, and preparation method and application thereof
A tetrahydropyridine and compound technology, which is applied in the field of preparation of antitumor drugs, can solve the problems of short action time and half-life in vivo, low bioavailability, weak selectivity and inhibitory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 2-chloro 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidinyl)-(2-fluoro-5-[(4-oxo-3,4-dihydrodi Preparation of azinaphthalen-1-yl)methyl]phenyl)methanone
[0024]
[0025] 5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoic acid was purchased from Shanghai Bobang Pharmaceutical Technology Co., Ltd., 2-chloro-5,6,7, References for the synthesis of 8-tetrahydropyrido[4,3-d]pyrimidine hydrochloride European Journal of Medicinal Chemistry.2014,79,399-412.
[0026] Dissolve 0.05g (0.1508mmol) of 2-fluoro-5-[(4-oxo-3,4-dihydronaphthalene-1-yl)methyl]benzoic acid in 5ml of dichloromethane, and add EDCI 0.0516g (0.268mmol) and HOBT 0.0362g (0.268mmol), add a few drops of DMF to help dissolve. After stirring at room temperature for 1 h, DIEA (1.88ml, 13,4mmol) was added, and 0.551g (0.268mmol) of 2-chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine hydrochloride was added ), continue stirring at room temperature for 24h. The reaction solution was washed three time...
Embodiment 2
[0028] (2-(1-Methyl-piperazinyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidinyl)-(2-fluoro-5-[(4-oxo Preparation of -3,4-dihydronaphthalene-1-yl)methyl]phenyl)methanone
[0029]
[0030] 2-Chloro-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidinyl)-(2-fluoro-5-[(4-oxo-3,4-dihydrodiazepine Naphthalene-1-yl)methyl]phenyl)methanone (50mg 0.111mmol 449) was dissolved in 10ml anhydrous THF (plus 2ml DMF co-solvent), N-methylpiperazine (22.29mg 0.22mmol 100.1) and three Ethylamine (0.046ml 0.33mmol 101.19) was reacted overnight at 60°C. The reaction solution was spin-dried and dispersed in 20 ml of ethyl acetate, washed with 20 ml of water and saturated sodium chloride solution successively, and the ethyl acetate layer was dried over anhydrous sodium sulfate. Column chromatography: (petroleum ether: ethyl acetate = 1:2), 12.5 mg of white solid was obtained. Yield 22%. Mp: 154.5-156. 1H NMR (400MHz, Methanol-d 4 )δ8.37(tt,1H),8.20(s,1H),8.02–7.78(m,4H),7.51(dtd,1H),7.42(dd,J=6.4,2.3Hz,1H)...
Embodiment 3
[0032] (2-(1-piperidinyl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidinyl)-(2-fluoro-5-[(4-oxo-3, Preparation of 4-dihydronaphthalene-1-yl)methyl]phenyl)methanone
[0033]
[0034] This product consists of 2-chloro 5,6,7,8-tetrahydropyrido[4,3-d]pyrimidinyl)-(2-fluoro-5-[(4-oxo-3,4-dihydrodi Azanaphthalen-1-yl)methyl]phenyl)methanone (50mg 0.111mmol 449) and piperidine (18.9mg 0.22mmol 85.15) were prepared by the same preparation method as in Experimental Example 2 to obtain 28.3mg of white solid. Yield 51%. Mp: 119.9-121.1°C.
[0035] 1H NMR (400MHz, Chloroform-d) δ11.20(d, J=9.2Hz, 1H), 8.49(q, J=4.2, 3.8Hz, 1H), 8.14(s, 1H), 8.05–7.55(m, 3H),7.58–7.17(m,2H),7.07(d,J=3.4Hz,1H),4.74(s,1H),4.31(d,J=7.1Hz,2H),4.27–3.83(m,1H ), 3.77(t, J=5.3Hz, 3H), 3.57(t, J=5.9Hz, 1H), 2.88(s, 2H), 2.00(d, J=46.1Hz, 2H), 1.77–1.50(m ,4H),1.53–0.62(m,2H).MS(ESI),[M+H] + 499.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com