Organic photoelectric material and application thereof
A technology of organic optoelectronic materials and electroluminescent devices, applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the efficiency roll-off, low S1 state radiation transition rate, difficult and high exciton utilization rate and high fluorescence radiation efficiency, etc. problems, to achieve the effect of improved performance, excellent fluorescence emission capability, and wide spectral coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
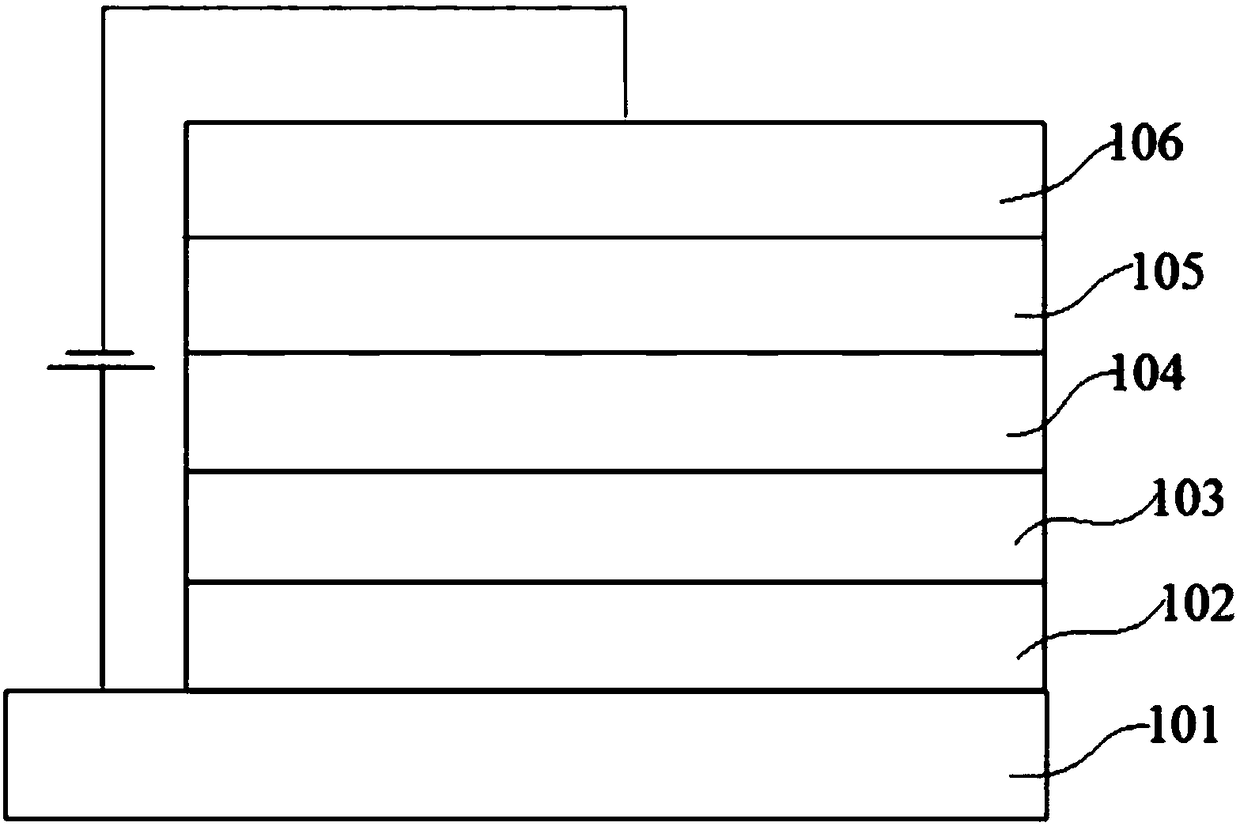
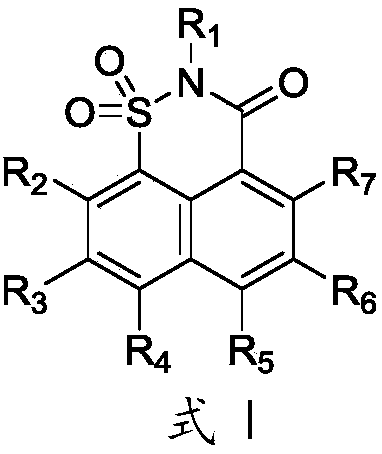
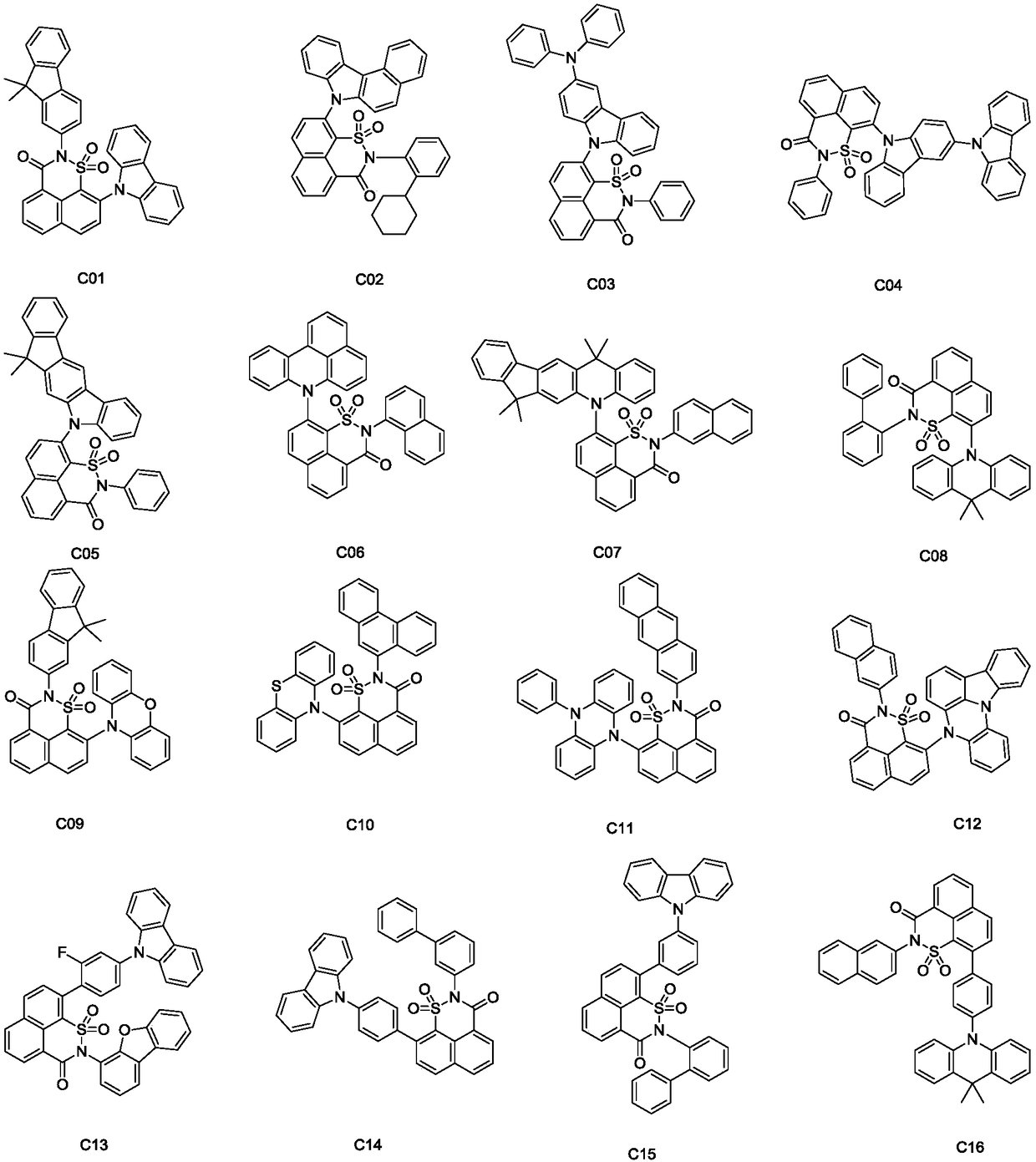
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 compound C01
[0035] (1) Preparation of compound C01-A
[0036]
[0037]Add 1-naphthoic acid 8-sulfonic acid cyclic anhydride (23.4g, 0.1mol), 9,9-dimethyl-2-aminofluorene (25.1g, 0.12mol) and glacial acetic acid (300mL) into a 1L three-necked flask, Raise the temperature to reflux, keep warm for 12 hours under nitrogen atmosphere, then lower to 20-25°C, pour the reaction solution into 1000mL deionized ice water, stir and react for 30min, filter with suction, and use 500mL deionized water to wash and filter Cake, then select 200mL absolute ethanol to reflux for beating, cool down to 20-25°C, filter with suction, collect the filter cake, obtain C01-A as 31.5g, and the calculated yield is 74.10%, mass spectrometry detection (abbreviated as MS) (m / z ): 425.15.
[0038] (2) Preparation of compound C01-B
[0039]
[0040] Add C01-A (21.3g, 0.05mol) and THF (800mL) into a 2L three-necked flask, under a nitrogen atmosphere, control the...
Embodiment 2
[0044] The preparation of embodiment 2 compound C25
[0045] (1) Preparation of compound C25-A
[0046]
[0047] Add 1-naphthoic acid 8-sulfonic acid cyclic anhydride (23.4g, 0.1mol), aniline (10.2g, 0.11mol) and glacial acetic acid (150mL) into a 500mL three-necked flask, heat up to reflux, and keep the reaction under nitrogen atmosphere After 9 hours, lower the temperature to 20-25°C, pour the reaction solution into 1000mL of deionized ice water, stir for 30min, then filter with suction, rinse the filter cake with 150mL of deionized water, and then use 100mL of absolute ethanol to reflux for beating. Cool down to 20-25°C, filter with suction, and collect the filter cake to obtain 23.3 g of C25-A, with a calculated yield of 75.48%. Mass spectrometry (abbreviated as MS) (m / z): 309.06.
[0048] (2) Preparation of compound C25-B
[0049]
[0050] Add C25-A (15.5g, 0.05mol) and THF (400mL) into a 1L three-necked flask. L), keep the reaction for 30 minutes after dropping,...
Embodiment 3
[0054] The preparation of embodiment 3 compound C38
[0055] (1) Preparation of compound C38-A
[0056]
[0057] Add C25-A (12.4g, 0.04mol) and THF (400mL) into a 1L three-necked flask. L), keep the temperature for 1.0 hours after the dropping, control the internal temperature to be less than -78°C, add dibromoethane (9.4g, 0.05mol) dropwise, and keep the temperature for 1.0 hrs after the dropping. Once again, control the internal temperature to less than -70°C, slowly add n-butyllithium n-hexane solution (18mL, 2.5mol / L) dropwise, and keep it warm for 1.0h after the dropping, and control the internal temperature to less than -70°C, add dibromoethane dropwise (9.4g, 0.05mol), after dripping, keep warm for 1.0hrs, slowly heat up to 15-20°C, add 200mL of deionized solution to the reaction solution, stir the reaction for 30min to quench the reaction, remove the solvent under reduced pressure until there is no fraction, Add 300mL of deionized water to stir at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com