A substituted boronic acid compound, a pharmaceutical composition comprising the compound and its use
A compound and composition technology, applied in the field of medicine, can solve the problems of fast clearance rate, short half-life, low in vivo stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
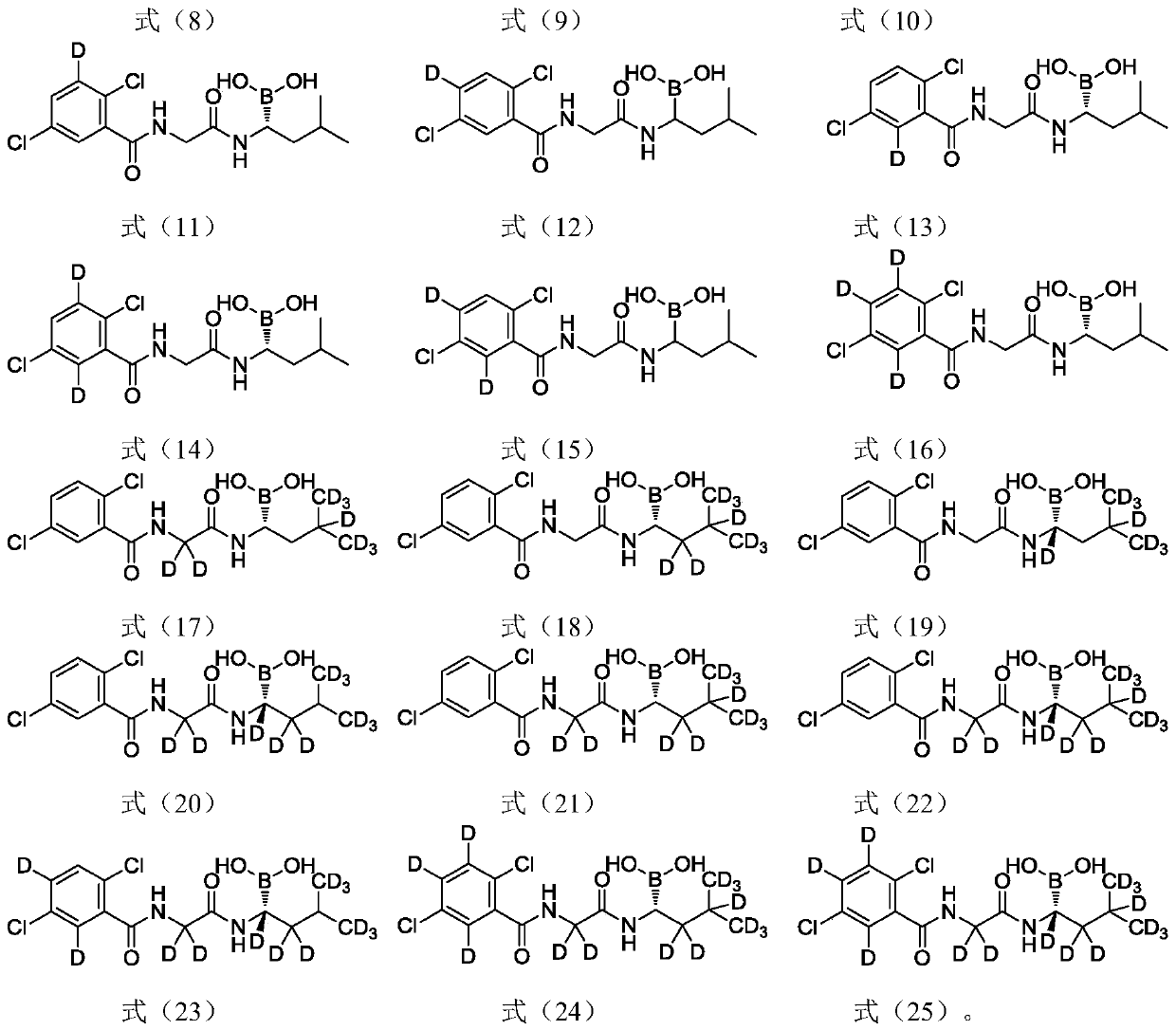
[0044] Example 1 Preparation of (R)-(1-(2-(2,5-dichlorobenzamido)-2,2-d2-acetamido)-3-methylbutyl base) boronic acid (compound I-1)
[0045]
[0046] Concrete synthetic steps are as follows:
[0047]
[0048] Step 1: Synthesis of 2,2-d2-glycine (Compound 3).
[0049] Add glycine (200mg, 2.66mmol) and salicylaldehyde (0.04mL, 0.376mmol) into the reaction flask, add 10mL of deuterated acetic acid to dissolve, heat to 100°C under nitrogen protection, stir for 2 hours, cool to room temperature, concentrate to remove acetic acid Then add 2 mL of heavy water, stir at room temperature for 15 minutes, then add a small amount of water to dilute, add activated carbon for decolorization for 0.5 hours, filter, and concentrate the filtrate to dryness. Add a small amount of methanol, stir to precipitate a white solid, filter, and vacuum dry to obtain 176.7 mg of the product, yield: 88%. LC-MS(APCI):m / z=78.1(M+1) + .
[0050] Step 2: Synthesis of 2,5-[(dichlorobenzoyl)amino]-d2-...
Embodiment 2
[0064] Example 2 Preparation of (R)-(1-(2-(2,5-dichlorobenzamido)acetamido)-1-d-3-methylbutyl)boron Acid (Compound I-2)
[0065]
[0066] Concrete synthetic steps are as follows:
[0067]
[0068] Step 1: Synthesis of 2,5-[(dichlorobenzoyl)amino]acetic acid (compound 13).
[0069] Add glycine (1.125g, 15mmol) and sodium hydroxide (750mg, 18.75mmol) to the reaction flask, add 7.5mL water to dissolve, add dropwise 2,5-dichlorobenzoyl chloride (623.7mg, 3.0mmol) under ice bath 1mL tetrahydrofuran solution, the addition was completed, and the reaction was stirred for 1 hour. After the reaction was detected by TLC, the pH was adjusted to acidic with dilute hydrochloric acid, and a white solid was precipitated, filtered, washed with ice water, and vacuum-dried to obtain 675 mg of the product, with a yield of 91.1%. LC-MS(APCI):m / z=248.3(M+1) + .
[0070] Step 2: Synthesis of (S)-1-chloro-1-d-3-methylbutylboronic acid-(+)-pinanediol ester (compound 14).
[0071] Add co...
Embodiment 3
[0080] Example 3 Preparation of (R)-(1-(2-(2,5-dichlorobenzamido)acetamido)-2,2-d2-3-methylbutyl) Boronic acid (Compound I-3)
[0081]
[0082] Concrete synthetic steps are as follows:
[0083]
[0084] Step 1: Synthesis of 1,1-d2-2-methylpropanol (compound 19).
[0085] Ethyl isobutyrate (580.8 mg, 5 mmol) was dissolved in anhydrous THF (20 mL), and LiAlD was added in portions under ice-cooling 4 (230.9mg, 5.5mmol), after the addition, it was raised to room temperature and reacted overnight. After the reaction was detected by TLC, sodium sulfate decahydrate was added under ice bath to quench the reaction, filtered to remove insoluble matter, and the filtrate was concentrated to obtain 246mg of product, yield 64.73% . Jump straight into the next step.
[0086] Step 2: Synthesis of 1,1-d2-bromoisobutane (compound 20).
[0087] Compound 19 (850 mg, 11.17 mmol) and carbon tetrabromide (3.7 g, 11.17 mmol) were dissolved in dichloromethane (15 mL), and triphenylphosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com