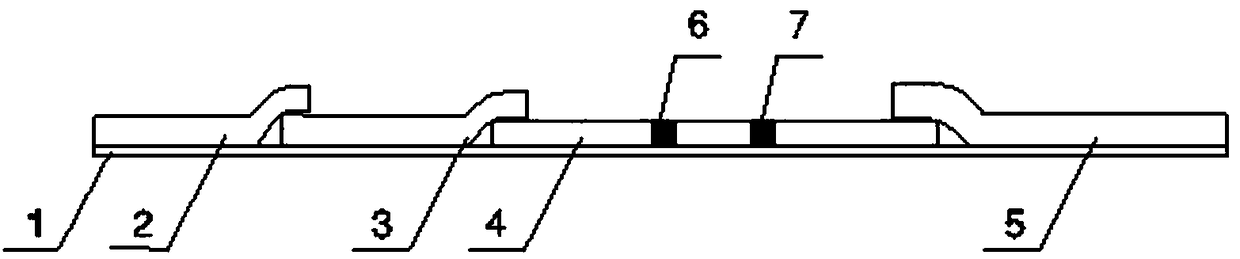
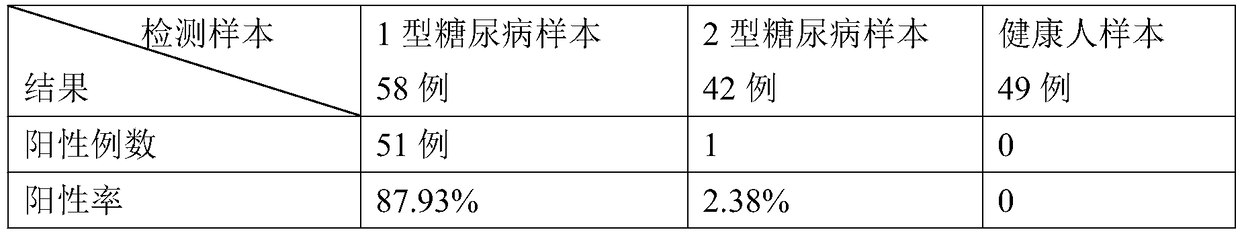
Quantum dot fluorescence chromatography immunization analysis reagent for detecting human blood ZnT8A
An immunoassay and fluorescence chromatography technology, applied in the fields of immunoassay and medical testing, can solve the problems of inability to judge the degree of pancreatic β-cell destruction, the severity of autoimmune diseases, the inability to accurately quantitatively measure autoantibodies, and the cumbersome operation methods, etc. Low, easy to operate, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for preparing a quantum dot fluorescence chromatography immunoassay reagent for detecting ZnT8A in human blood, comprising the steps of:
[0042] Preparation of sample pad: use 0.01M Tris-HCl with pH=7.2 as solvent, make anti-erythrocyte antibody into 0.15mg / ml solution, and press 35ul / cm 2 Spray the erythrocyte antibody solution onto a 1.2cm×30cm polyester fiber membrane, and dry it for later use;
[0043] Preparation of quantum dot marker-conjugated pads:
[0044] 1) Take 50ul, 10mg / mL quantum dots (the diameter of the quantum dots is 3nm), add 100ul 20mM PBS with pH=7.2, then add 15μL 25% glutaraldehyde, mix well, stir and activate at room temperature for 5 hours;
[0045] 2) Dialyze the liquid obtained in step 1) with 10-50 mM PBS at pH=7.2 for 22 hours, and change the liquid twice;
[0046] 3) Mix the mouse anti-human IgG antibody with 20mM PBS pH=7.2 to 4mg / mL, take 20μL and add it to the liquid obtained in step 2), mix well, stir and react at 5°C for 1...
Embodiment 2
[0058] A method for preparing a quantum dot fluorescence chromatography immunoassay reagent for detecting ZnT8A in human blood, comprising the steps of:
[0059] Preparation of sample pad: use 0.01M Tris-HCl with pH=7.2 as solvent, make anti-erythrocyte antibody into 0.2mg / ml solution, and press 20ul / cm 2 Spray the erythrocyte antibody solution onto the polyester fiber membrane, and let it dry for later use;
[0060] Preparation of quantum dot marker-conjugated pads:
[0061] 1) Take 50ul, 5mg / mL quantum dots (the diameter of the quantum dots is 2nm), add 100ul 20mM PBS with pH=7.2, then add 10μL 25% glutaraldehyde, mix well, stir and activate at room temperature for 6 hours;
[0062] 2) The liquid obtained in step 1) was dialyzed with 50 mM PBS with pH=7.2 for 20 hours, and the liquid was changed 3 times;
[0063] 3) Mix the mouse anti-human IgG antibody with 20mM PBS pH=7.2 to 3mg / mL, take 20μL and add it to the liquid obtained in step 2), mix well, stir and react at 2°C f...
Embodiment 3
[0074] A method for preparing a quantum dot fluorescence chromatography immunoassay reagent for detecting ZnT8A in human blood, comprising the steps of:
[0075] Preparation of sample pad: use 0.01M Tris-HCl with pH=7.2 as solvent, make anti-erythrocyte antibody into 0.1mg / ml solution, and press 50ul / cm 2 Spray the erythrocyte antibody solution onto the polyester fiber membrane, and let it dry for later use;
[0076] Preparation of quantum dot marker-conjugated pads:
[0077] 1) Take 50ul, 20mg / mL quantum dots (the diameter of the quantum dots is 4nm), add 100ul 20mM PBS with pH=7.2, then add 20μL 25% glutaraldehyde, mix well, stir and activate at room temperature for 3 hours;
[0078] 2) The liquid obtained in step 1) was dialyzed with 10 mM PBS with pH=7.2 for 24 hours, and the liquid was changed twice;
[0079] 3) Mix the mouse anti-human IgG antibody with 20mM PBS pH=7.2 to 5mg / mL, take 20μL and add it to the liquid obtained in step 2), mix well, stir and react at 8°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com