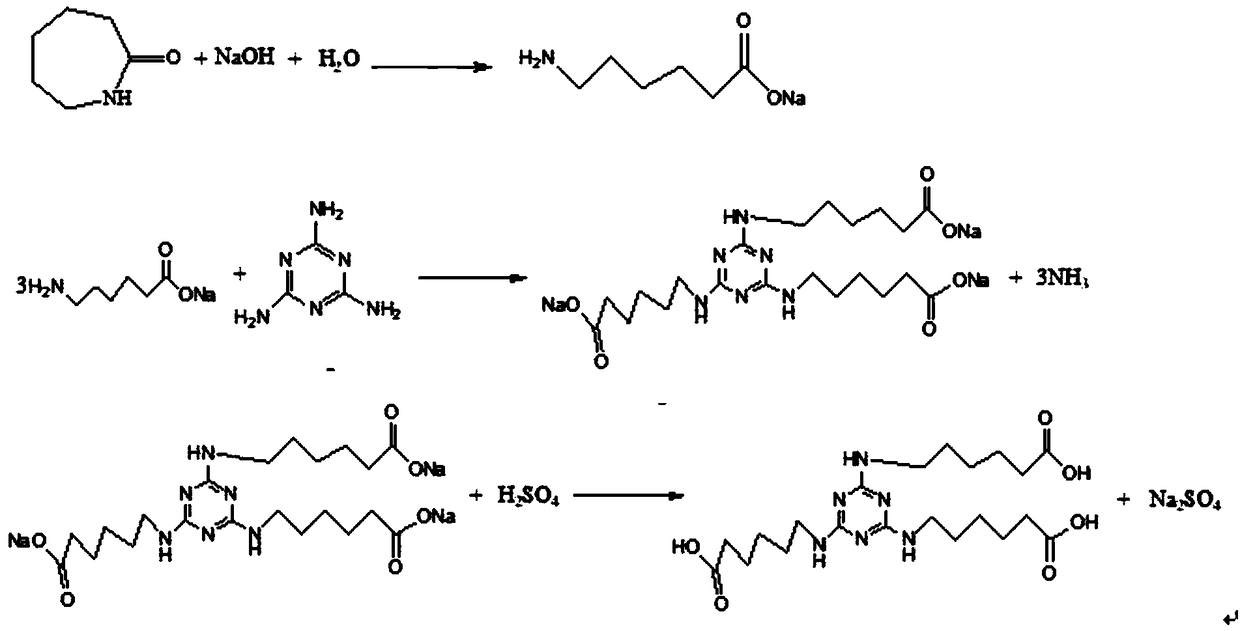
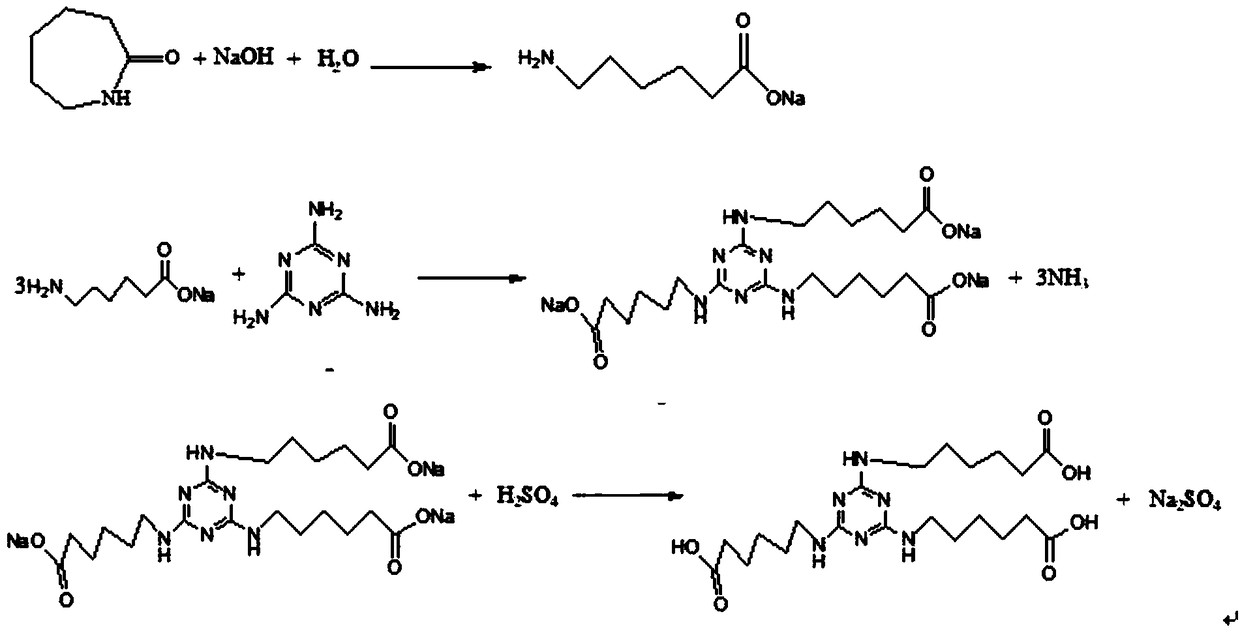
Novel synthesis method of 2,4,6-tri(aminocaproate)-1,3,5-triazine
A synthesis method, the technology of aminocaproic acid, applied in the field of synthesis of 2,4,6-tri-1,3,5-triazine, to achieve the effects of less product loss, lessen the environmental protection pressure of enterprises, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Add 339g caprolactam, 450g cesium hydroxide and 1500g solvent xylene into the bottle. 54g of water was added dropwise with stirring, the reaction exothermed, and the temperature was controlled not to exceed 90°C. The reaction was kept at 80-90°C for 3 hours, and gas chromatography (GC) analysis showed that the caprolactam conversion rate was greater than 99.5%, and the reaction was completed. The temperature was lowered to 40°C, 126g of melamine was added, and the mixture was stirred and heated. When the temperature rises to 90-100°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to 110-120°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to At 135-145°C, gaseous ammonia is released, absorbed by water, and kept at 135-145°C to react until the raw material melamine and intermediates disappeared by high performance liquid chromatography (HPLC) analysis, the ...
example 2
[0026] Add 339g caprolactam, 224g potassium hydroxide and 1000g solvent dichlorobenzene into the bottle. 54g of water was added dropwise with stirring, the reaction exothermed, and the temperature was controlled not to exceed 90°C. The reaction was kept at 80-90°C for 3 hours. GC analysis showed that the conversion rate of caprolactam was greater than 99.5%, and the reaction was completed. The temperature was lowered to 40°C, 126g of melamine was added, and the mixture was stirred and heated. When the temperature rises to 90-100°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to 110-120°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to At 135-145°C, gaseous ammonia is released, absorbed by water, and reacted at 135-145°C at 135-145°C until the raw material melamine and intermediates disappeared as analyzed by HPLC, the reaction ends. Cool down to below 40°C, ...
example 3
[0028] Add 339 g caprolactam, 200 g sodium hydroxide and 800 g solvent xylene into the bottle. 54g of water was added dropwise with stirring, the reaction exothermed, and the temperature was controlled not to exceed 90°C. The reaction was kept at 80-90°C for 3 hours. GC analysis showed that the conversion rate of caprolactam was greater than 99.5%, and the reaction was completed. The temperature was lowered to 40°C, 126g of melamine was added, and the mixture was stirred and heated. When the temperature rises to 90-100°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to 110-120°C, gaseous ammonia is released, absorbed by water, and the gas release is not obvious, continue to heat up to At 135-145°C, gaseous ammonia is released, absorbed by water, and reacted at 135-145°C at 135-145°C until the raw material melamine and intermediates disappeared as analyzed by HPLC, the reaction ends. Cool down to below 40°C, stir slowl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com