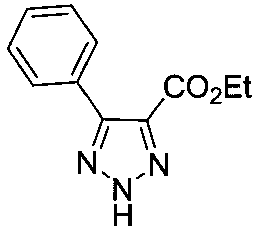
Method of preparing 4,5-disubstituted 1,2,3-triazole with pyridinium salt
A technology of triazole and disubstituted, which is applied in the field of preparation of 1,2,3-triazole compounds, can solve the problems of difficulty in synthesizing olefins and cannot be purchased, and achieves the effects of simple synthesis operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Ethyl α-bromoacetate (200mg, 1.2mmol) and pyridine (138mg, 1.8mmol) were dissolved in DMSO (5mL), stirred at room temperature for 5 hours. Then add benzaldehyde (191mg, 1.8mmol), NaN 3 (117mg, 1.8mmol) and L-proline (14mg, 0.12mmol), the reaction was stirred at room temperature. After the reaction was completed, the reaction solution was poured into ice water and extracted with ethyl acetate (20mL×4). The organic phases were combined, dried, and the solvent was removed under reduced pressure to obtain a crude product, which was separated and purified with a silica gel column to obtain 207 mg of the product, with a yield of 96%.
Embodiment 2
[0024] Ethyl α-bromoacetate (200mg, 1.2mmol) and pyridine (138mg, 1.8mmol) were dissolved in DMSO (5mL), stirred at room temperature for 5 hours. Then add benzaldehyde (191mg, 1.8mmol), NaN 3 (117mg, 1.8mmol) and serine (15mg, 0.12mmol), the reaction was stirred at room temperature. After the reaction was complete, the reaction solution was poured into ice water and extracted with ethyl acetate (20mL×4). The organic phases were combined, dried, and the solvent was removed under reduced pressure to obtain a crude product, which was separated and purified with a silica gel column to obtain 245mg of the product, with a yield of 94%.
Embodiment 3
[0026] Ethyl α-bromoacetate (200mg, 1.2mmol) and pyridine (138mg, 1.8mmol) were dissolved in DMSO (5mL), stirred at room temperature for 5 hours. Then add benzaldehyde (191mg, 1.8mmol), NaN 3 (117mg, 1.8mmol) and glycine (11mg, 0.12mmol), stirred at room temperature. After the reaction was completed, the reaction solution was poured into ice water and extracted with ethyl acetate (20mL×4). The organic phases were combined, dried and the solvent was removed under reduced pressure to obtain a crude product, which was separated and purified with a silica gel column to obtain 244 mg of the product, with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com