Preparation method of 3-substituted dimethyl glutarate and dimethyl glutaconate
A technology of glutaric acid diester and glutaric acid diester is applied in the field of preparation of 3-substituted glutaric acid diester and glutaric acid diester, and can solve the problem of high equipment requirements, inconvenient use, use of hydrogen, etc. problems, to achieve the effect of low requirements for preparation conditions, wide market sources and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
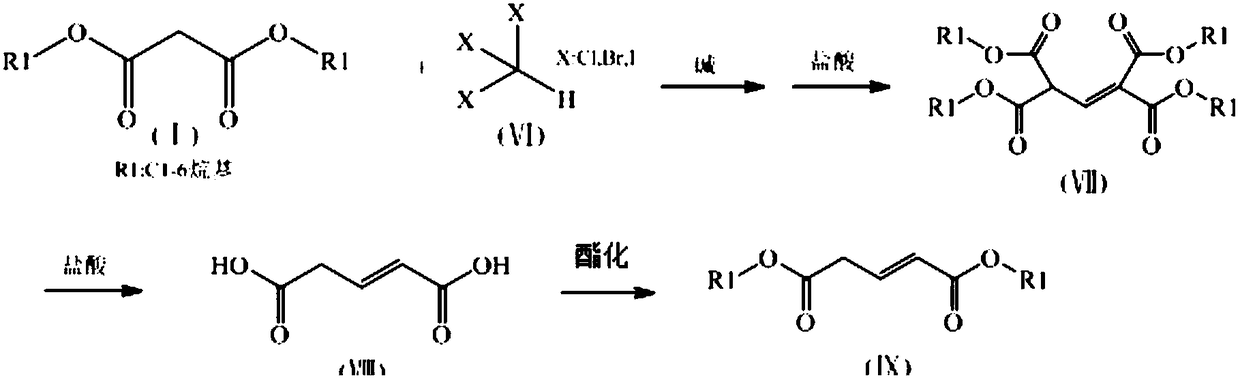
[0029] Preparation of methyl 3-methoxyglutarate
[0030] Add dimethyl malonate (13.2kg, 0.10kmol), methanol (16kg), and sodium methoxide (6.48kg, 0.12kmol) to a 50L enamel reactor equipped with stirring and a thermometer, and react at 70°C for 1 hour , then heated to reflux, added methoxydibromomethane (9.80kg, 0.048kmol), reacted for 2 hours and cooled to 0°C, filtered, the filter cake was washed with 5kg ice methanol, the resulting solid was added in concentrated hydrochloric acid in batches, and the addition was complete Raise the temperature to 90°C, cool down to 25°C after reacting for 3 hours, extract with ethyl acetate (20kg x 3 times), combine the oil phases, and precipitate under reduced pressure to obtain 7.20kg of 3-methoxyglutaric acid with a yield of 92.5 %, content 98.5%.
[0031] Add methanol (10.0kg, 3.1kmol), 3-methoxyglutaric acid (7.20kg, 0.044kmol) and concentrated sulfuric acid (72g) into a 50L enamel reactor equipped with stirring and a thermometer, and ...
Embodiment 2
[0033] Preparation of dimethyl glutaconate
[0034] Add dimethyl malonate (13.2kg, 0.1kmol), ethanol (32kg), sodium ethoxide (7.48kg, 0.11kmol) to a 100L enamel reactor equipped with stirring and a thermometer, and react at 70°C for 1 hour , then heated to reflux, added chloroform (5.70kg, 0.048kmol), reflux reaction for 2 hours and then cooled to 0°C, filtered, the filter cake was washed with 5kg ice ethanol, the resulting solid was added in concentrated hydrochloric acid in batches, and the temperature was raised after the addition was completed to 90°C, reacted for 3 hours and then cooled to 25°C, extracted with ethyl acetate (20kg×3 times), combined the oil phases, and desolvated under reduced pressure to obtain 5.57kg of glutaconic acid with a yield of 89.2% and a content of 98.0% .
[0035] Add methanol (10.0kg, 3.1kmol), glutaconate (5.57kg, 0.043kmol) and concentrated sulfuric acid (72.0g) into a 20L enamel reactor equipped with stirring and a thermometer, and heat to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com