Agent for suppressing odors of polysulfide compounds
一种多硫化物、抑制剂的技术,应用在烃类化合物有效成分、含有效成分的医用配制品、除臭等方向,能够解决尚未见报导等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
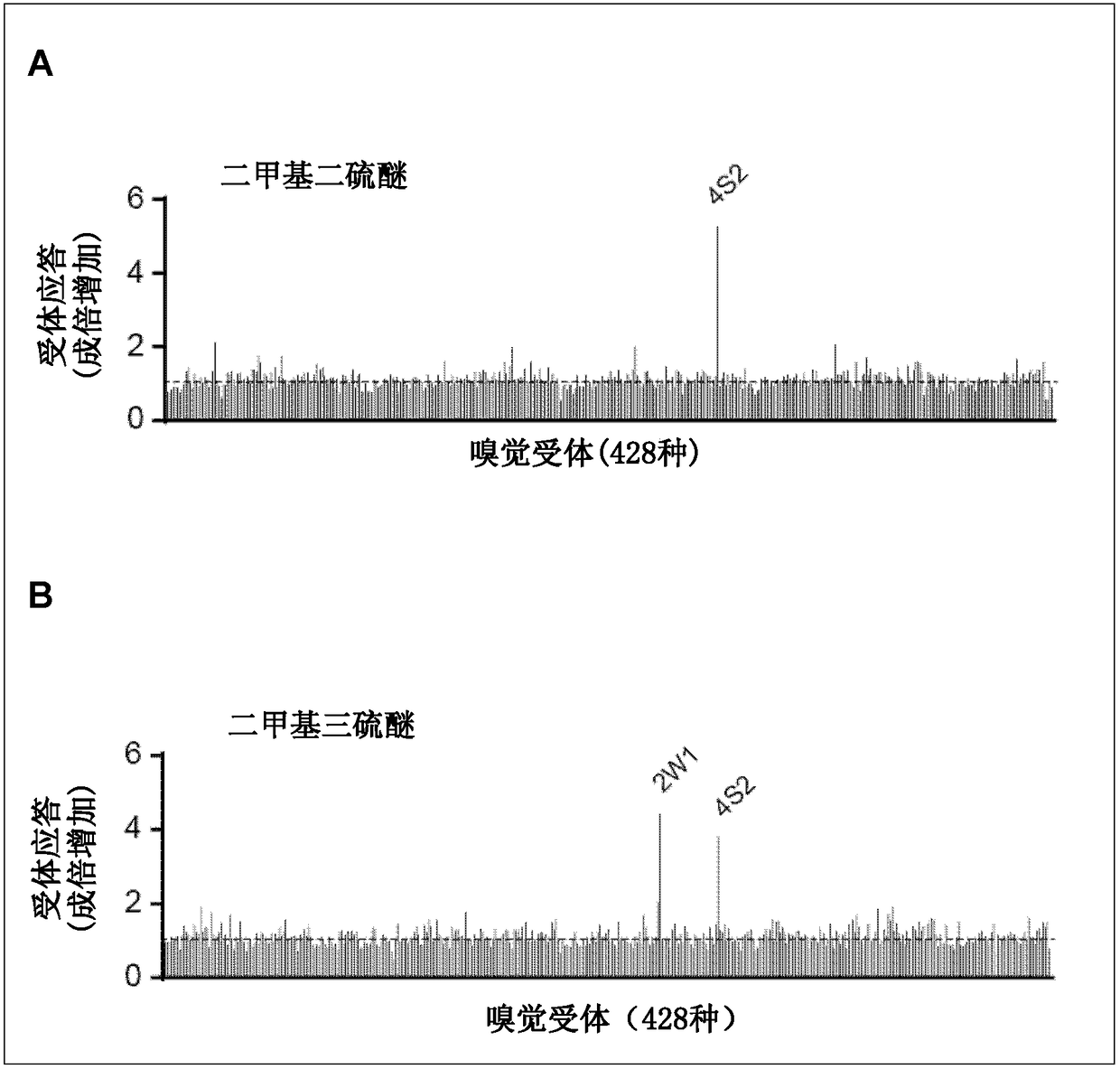
[0102] Example 1 Identification of Olfactory Receptors Responsive to Polysulfides
[0103] (1) Cloning of human olfactory receptor gene
[0104] Based on the sequence information registered in GenBank, human olfactory receptors were cloned by PCR using human genomic DNA female (G1521: Promega) as a template. According to the manual, each gene amplified by PCR method was introduced into the pENTR vector (Invitrogen). Click to reorganize.
[0105] (2) Production of pME18S-human RTP1S vector
[0106] A vector expressing a protein receptor-transporting protein (receptor-transporting protein, RTP) that promotes cell membrane expression of an olfactory receptor polypeptide was prepared. Specifically, the gene encoding human RTP1S (GI: 50234917) was introduced into the EcoRI and XhoI sites of the pME18S vector.
[0107] (3) Production of olfactory receptor expressing cells
[0108] HEK293 cells expressing any one of the human olfactory receptors were produced. A reaction soluti...
Embodiment 2
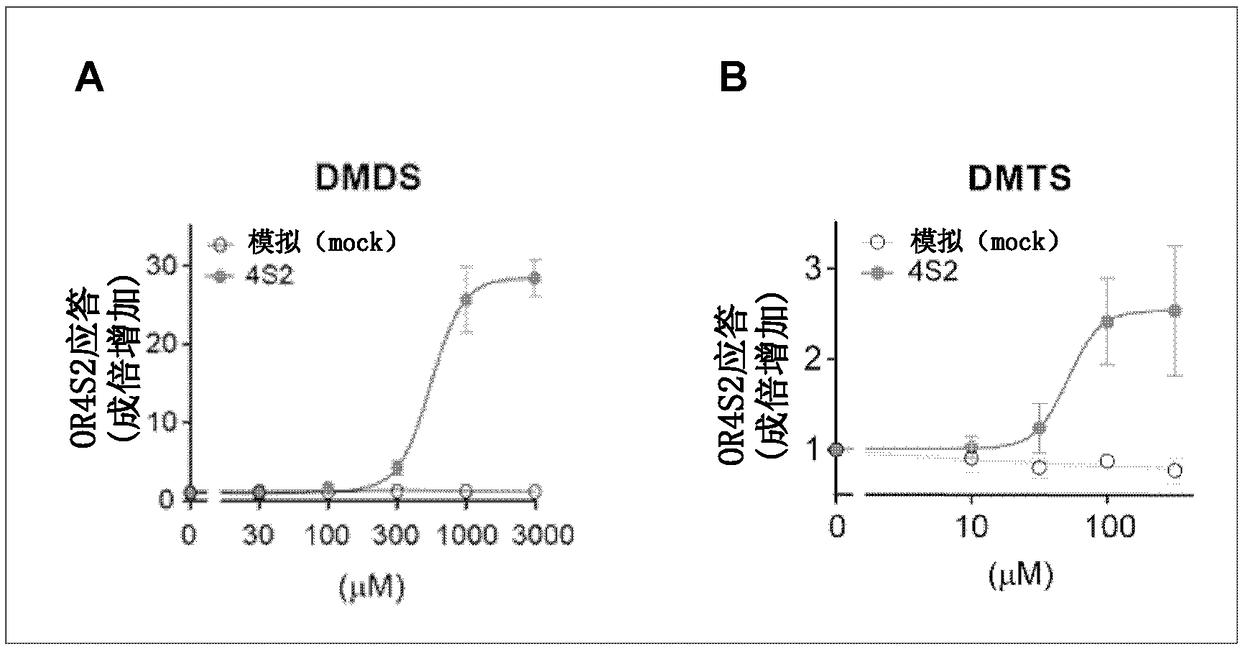
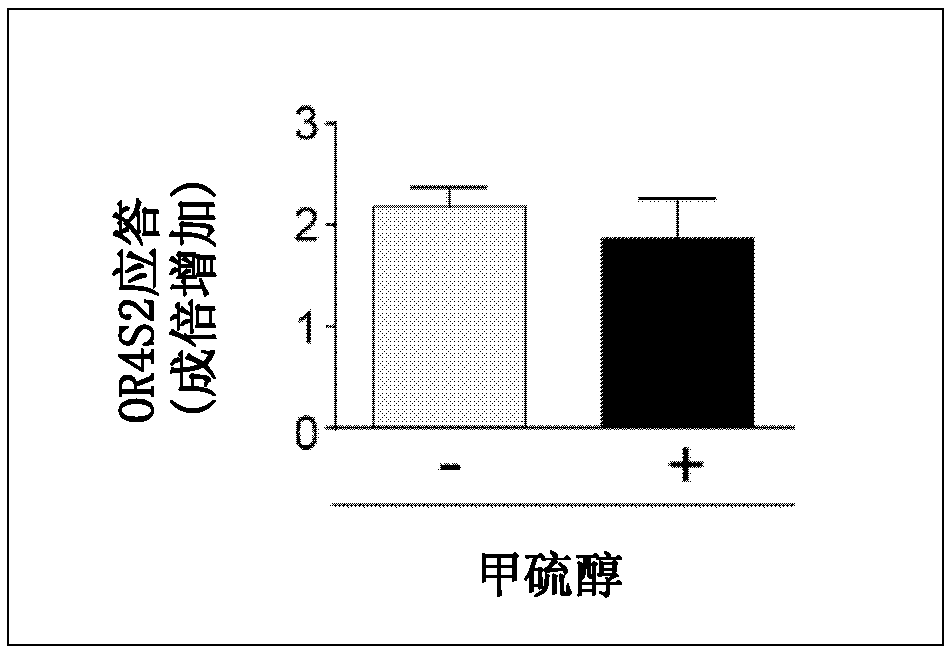
[0116] Exploration of embodiment 2 OR4S2 antagonists
[0117] HEK293 cells expressing OR4S2 (SEQ ID NO: 1) were produced by the same procedure as in Example 1 (1) to (3). Following the procedure of Example 1 (4), the response of the olfactory receptors to DMDS (fLuc / hRluc value) in the presence and absence of the test substance was measured using the luciferase reporter gene assay. Obtain the fLuc / hRluc value (X) induced by DMDS stimulation alone, the fLuc / hRluc value (Y) of cells not stimulated by DMDS, the fLuc / hRluc value (Z) induced by the co-stimulation of DMDS and test substances, by The following calculation formula calculates the DMDS response intensity (Response (%)) of the receptor in the presence of the test substance.
[0118] Response(%)=(Z-Y) / (X-Y)×100
[0119] Three independent experiments were carried out, and the average value of each experiment was calculated. The added concentration of DMDS in the culture was 1 mM, and the added concentration of the test ...
Embodiment 3
[0122] The inhibitory ability of the odor of the polysulfide of embodiment 3OR4S2 antagonist
[0123] Confirmation of odor suppressing effect of polysulfides of trimethylhexanal, (THA), cis-4-heptenal and 1,4-cineole as OR4S2 antagonists identified in Example 2 by sensory test .
[0124] For DMDS, THA, cis-4-heptenal or 1,4-cineole, 0.1% (v / v) solutions in mineral oil were prepared. For DMTS, a 0.01% (v / v) solution in mineral oil was prepared. Put 2 cotton balls into a 20mL glass bottle (MARUEMU, No.6), one cotton ball is soaked in 30 μL of DMDS or DMTS solution, and the other cotton ball is soaked in any of the above three OR4S2 antagonists Solution 30μL. The glass bottle filled with the above-mentioned cotton balls was capped and allowed to stand at 37° C. for 1 hour, and then used as a test sample for a sensory test. A glass bottle containing only cotton balls containing a solution containing DMDS or DMTS was prepared as a reference sample, and a glass bottle containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com