In vitro culture medium and in vitro culture system for culturing human embryos
An in vitro culture and culture medium technology, applied in the direction of embryonic cells, culture process, tissue culture, etc., can solve the problems of high price, medium change in the middle, unsupported embryo growth and development, etc., to reduce the impact, improve the culture effect, reduce the The effect of the action steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] [Example 1] Embryo culture fluid formula and preparation method:
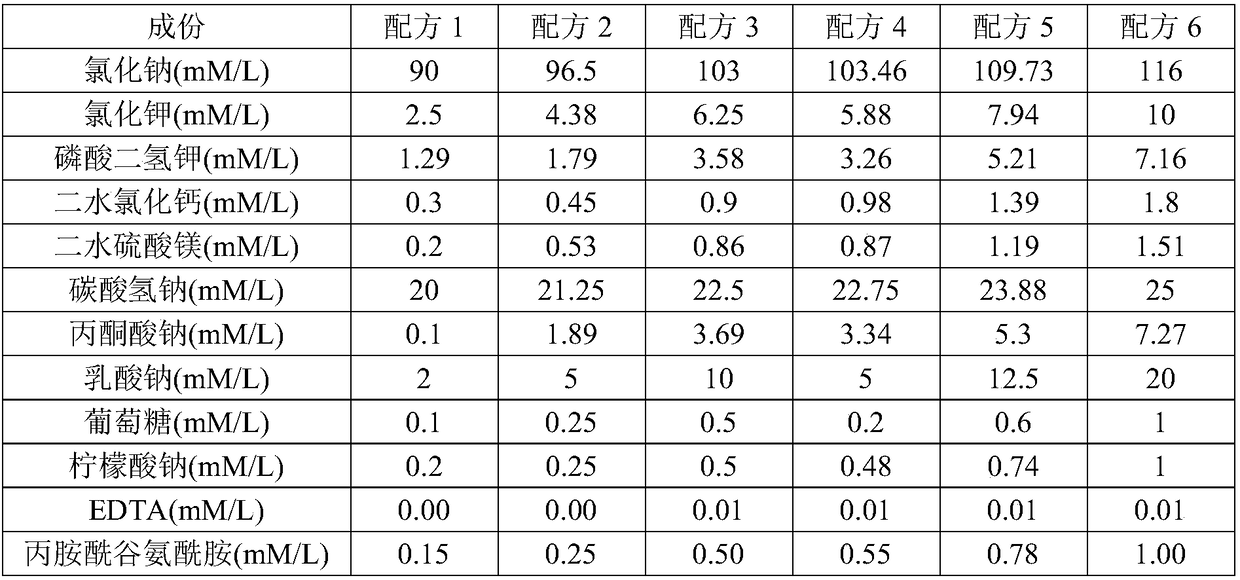
[0020] A. Culture medium formula:
[0021]
[0022]
[0023] B. Preparation method:
[0024] a. Sterilize the equipment used in the preparation at high temperature and dry it for later use. The whole preparation process is carried out in a clean workshop that meets the requirements;
[0025] b. Add the weighed ultrapure water, and then add sodium chloride, potassium chloride, potassium dihydrogen phosphate, calcium chloride dihydrate, magnesium sulfate dihydrate, Disodium EDTA, essential amino acids and non-essential amino acids, glucose, inositol, sodium citrate, taurine, sodium pyruvate, sodium lactate, start the agitator until the solid matter is completely dissolved, and make base liquid A;
[0026] c. Add the accurately weighed gentamicin sulfate and phenol red of each formula in the formula table to the above base liquid A, and finally add sodium bicarbonate, start the agitator until the so...
Embodiment 2
[0029] [Example 2] Detection of Embryo Culture Solution
[0030] pH detection
[0031] Take an appropriate amount of the sample to be tested and measure it three times with a pH meter, and take the average value of the three data obtained as the result, and it is considered qualified if it is 7.3±0.1.
[0032] Osmotic pressure test
[0033] After the osmometer is calibrated, take 50μl of the sample to be tested and put it into the test tube to start the test. When the value is stable, read the data, measure 3 data according to the above method, take the average as the result, and it is considered qualified if it is 257-273mOsm / kg .
[0034] Bacterial endotoxin detection
[0035] According to the requirements of the 2015 edition of the Pharmacopoeia of the People's Republic of China, the test is carried out by the limulus reagent gel method, and the result is ≤0.25EU / ml, which is considered qualified.
[0036] Cytotoxicity
[0037] Tested according to the provisions of GB / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com