A kind of functional group double-capped additive and its preparation and application
A functional group and double-capped technology, which is applied in the field of functional group double-capped additives and tire functional group double-capped additives, can solve problems such as deterioration, excess, and poor anti-aging performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] A preparation method of an additive with a functional group double-capped structure, comprising the following steps:
[0049] (1): take dicarboxylic acid and alkali, or directly take the salt of dicarboxylic acid and place it in a polar solvent, heat until the solution is completely transparent, and obtain solution A of the dicarboxylic acid salt product;
[0050] (2): Take functional group carboxylic acid and alkali, or place the salt of functional group carboxylic acid in a polar solvent, and heat until the solution is completely transparent to obtain solution B of functional group carboxylate product;
[0051] (3): under stirring condition, solution A, solution B are mixed with the metal M salt solution that configures, and react to obtain solid product, filter, wash, dry, promptly obtain the additive of target product functional group double-capping structure;
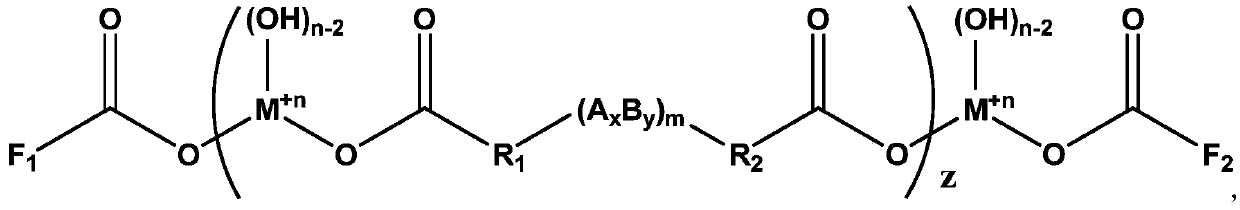
[0052] For example, the structural formula of the dicarboxylic acid can be: HOOC-R 1 -(A x B y ) m -R...
Embodiment 1
[0075] An additive with a functional group double-capped structure, its chemical structural formula is:
[0076]
[0077] The preparation method of above-mentioned compound, comprises the following steps:
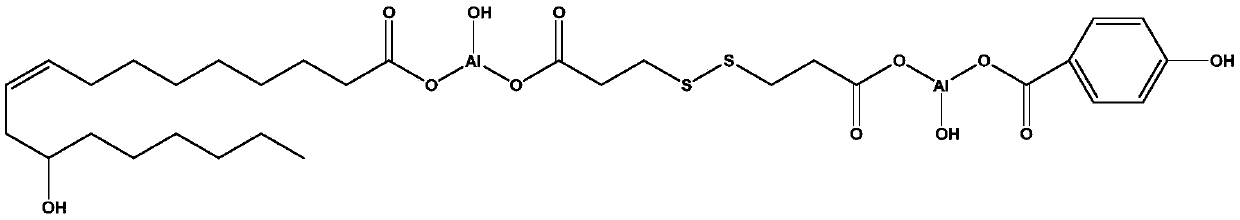
[0078] (1) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 42.05g of 3,3'-dithiodipropionic acid (purity≥99.0% (GC)(T), purchased from TCI) was added. The above mixture was heated to 90°C and stirred vigorously for 1 hour until the solution was completely transparent. (Solution A)
[0079] (2) Add 1 L of distilled water and 8 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 27.62g of 4-hydroxybenzoic acid (purity≥99%, purchased from Aladdin) and 64.09g of sodium ricinoleate (purity>90.0% (T), purchased from TCI) were added. The above mixture was heated to ...
Embodiment 2
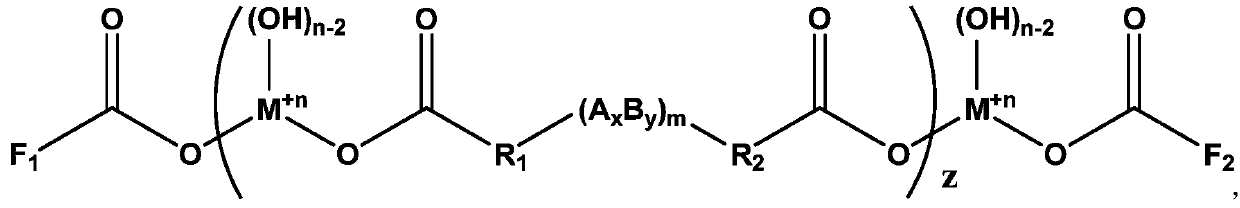
[0084] An additive with a functional group double-capped structure, its chemical structural formula is:
[0085]
[0086] The preparation method of above-mentioned compound, comprises the following steps:
[0087] (1) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 42.05g of 3,3'-dithiodipropionic acid (purity≥99.0% (GC)(T), purchased from TCI) was added. The above mixture was heated to 90°C and stirred vigorously for 1 hour until the solution was completely transparent. (Solution A)
[0088] (2) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide is completely dissolved, add 30.82g 3,4-dihydroxybenzoic acid (purity≥97%, purchased from Aladdin) and 60.10g 12-hydroxystearic acid (purity>80% (GC), purchased from TCI). The above mixture was heated to 90°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com