Additive with functional group double-terminated structure as well as preparation and application of additive
A functional group, double-end capping technology, applied in the field of additives with functional group double end capping structure and tire functional group double end capping structure, can solve the problems of high hysteresis loss, unsatisfactory effect, and poor anti-aging performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] A preparation method of an additive with a functional group double-capped structure, comprising the following steps:
[0049] (1): take dicarboxylic acid and alkali, or directly take the salt of dicarboxylic acid and place it in a polar solvent, heat until the solution is completely transparent, and obtain solution A of the dicarboxylic acid salt product;
[0050] (2): Take functional group carboxylic acid and alkali, or place the salt of functional group carboxylic acid in a polar solvent, and heat until the solution is completely transparent to obtain solution B of functional group carboxylate product;
[0051] (3): under stirring condition, solution A, solution B are mixed with the metal M salt solution that configures, and react to obtain solid product, filter, wash, dry, promptly obtain the additive of target product functional group double-capping structure;
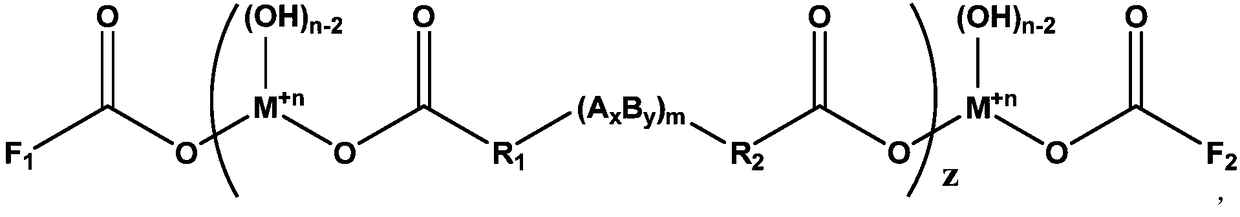
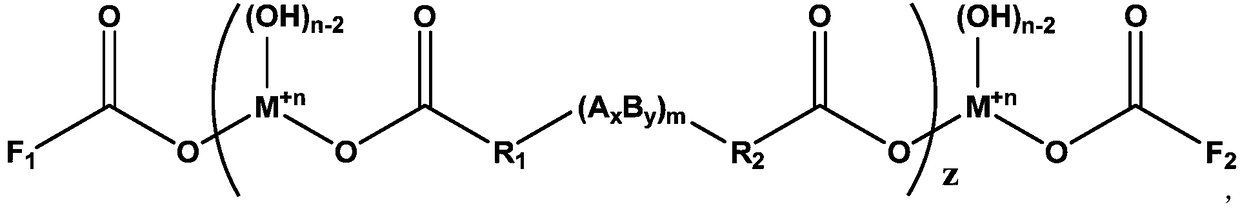
[0052] For example, the structural formula of the dicarboxylic acid can be: HOOC-R 1 -(A x B y ) m -R...
Embodiment 1
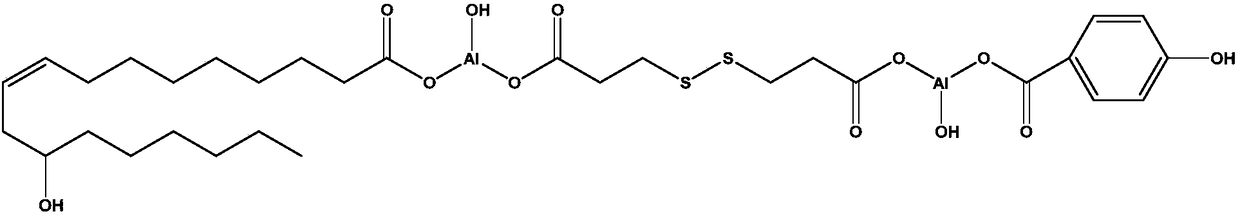
[0075] An additive with a functional group double-capped structure, its chemical structural formula is:
[0076]
[0077] The preparation method of above-mentioned compound, comprises the following steps:
[0078] (1) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 42.05g of 3,3'-dithiodipropionic acid (purity≥99.0% (GC)(T), purchased from TCI) was added. The above mixture was heated to 90°C and stirred vigorously for 1 hour until the solution was completely transparent. (Solution A)
[0079] (2) Add 1 L of distilled water and 8 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 27.62g of 4-hydroxybenzoic acid (purity≥99%, purchased from Aladdin) and 64.09g of sodium ricinoleate (purity>90.0% (T), purchased from TCI) were added. The above mixture was heated to ...
Embodiment 2
[0084] An additive with a functional group double-capped structure, its chemical structural formula is:
[0085]
[0086] The preparation method of above-mentioned compound, comprises the following steps:
[0087] (1) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide was completely dissolved, 42.05g of 3,3'-dithiodipropionic acid (purity≥99.0% (GC)(T), purchased from TCI) was added. The above mixture was heated to 90°C and stirred vigorously for 1 hour until the solution was completely transparent. (Solution A)
[0088] (2) Add 1 L of distilled water and 16 g of solid sodium hydroxide (purity ≥ 97%, purchased from Aladdin) into a 2 L beaker. After the sodium hydroxide is completely dissolved, add 30.82g 3,4-dihydroxybenzoic acid (purity≥97%, purchased from Aladdin) and 60.10g 12-hydroxystearic acid (purity>80% (GC), purchased from TCI). The above mixture was heated to 90°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Ring outer diameter | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com