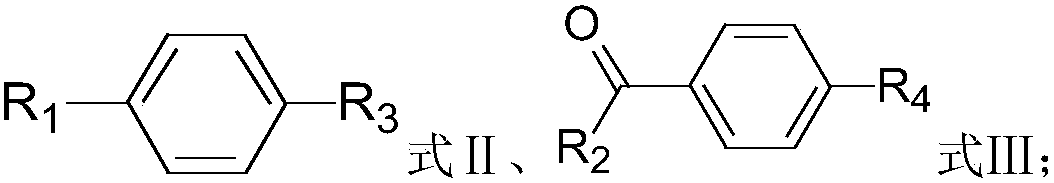Method for synthesizing substituted biphenyl compound
A synthesis method and compound technology are applied in the synthesis field of biphenyl compounds, which can solve the problems of complicated operation and high cost of the preparation method, and achieve the effects of low price, reduced material cost and enlarged production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In the 1000mL three-necked flask, add 4-(methylsulfonyl) phenylboronic acid (10.0g, 50mmol), 4'-bromo-2,2,2-trifluoroacetophenone (12.5g, 50mmol), 10% palladium carbon ( 0.5g), sodium carbonate (15.0g, 150mmol), 100mL each of methanol and water, vacuumize the reaction flask and fill with nitrogen, react at 30°C for 8h, TLC shows that all the raw materials have reacted, stop the reaction, add 200mL ethyl acetate to the reaction flask, Stir for 1 h, filter with suction, separate the upper ethyl acetate phase, and remove the solvent to obtain a total of 15.2 g of white solids, with a yield of 92.6%.
Embodiment 2
[0044] In the 1000mL three-necked flask, add 4-(methylsulfonyl) phenylboronic acid (10.0g, 50mmol), 4'-bromo-2,2,2-trifluoroacetophenone (12.5g, 50mmol), 10% palladium carbon ( 0.5g), sodium carbonate (15.0g, 150mmol), 100mL each of ethanol and water, vacuumize the reaction bottle and fill it with nitrogen, react at 30°C for 8h, TLC shows that all the raw materials have reacted, stop the reaction, add 200mL ethyl acetate to the reaction bottle, Stir for 1 h, filter with suction, separate the upper ethyl acetate phase, and remove the solvent to obtain 14.6 g of white solids, with a yield of 89.0%.
Embodiment 3
[0046] Add 4-(methylsulfonyl) phenylboronic acid pinacol ester (14.1g, 50mmol), 4'-bromo-2,2,2-trifluoroacetophenone (12.5g, 50mmol) successively in 1000mL three-necked flask, 10 % palladium carbon (0.5g), sodium carbonate (15.0g, 150mmol), 100mL each of methanol and water, vacuumize the reaction bottle and fill it with nitrogen, react at 30°C for 8h, TLC shows that all the raw materials have reacted, stop the reaction, add 200mL to the reaction bottle Ethyl acetate was stirred for 1 h, filtered with suction, the upper ethyl acetate phase was separated, and the solvent was removed to obtain a total of 14.2 g of white solids, with a yield of 86.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com