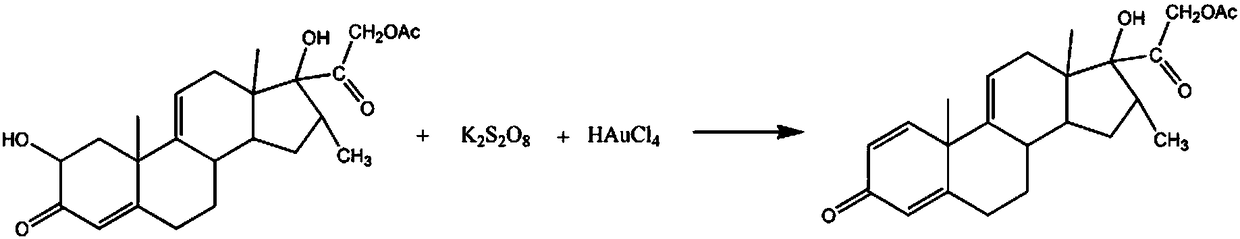
Synthetic method for pregnatriene-16alpha-methyl-17alpha,21-diol-3,20-dione-21-acetate
A synthetic method and acetate technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high heat resistance requirements for reaction equipment, increased manufacturing costs, and reduced reaction costs, so as to avoid the risk of poisoning and reduce the intermediate links of the reaction , the effect of reducing the reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthetic method of pharmaceutical intermediate 1,4,9(11)-pregnatriene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate comprises the following steps:
[0017] A: Add 3mol 2-hydroxyl-4,9(11)-pregnadiene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate in the reaction vessel, 900ml quality For a propionitrile solution with a fraction of 30%, raise the temperature of the solution to 50°C, add 3 mol of potassium peroxodisulfate, 1.2L of an oxalic acid solution with a mass fraction of 20%, and react for 40 minutes;
[0018] B: Raise the temperature of the solution to 60°C, add 3mol of chloroauric acid powder, 800ml of sodium sulfate solution with a mass fraction of 15%, continue the reaction for 2h, adjust the pH to 6, let it stand for 30min, and use 10% of the mass fraction to chlorinate Wash 3 times with sodium solution, wash 2 times with 70% ethylbenzene solution, recrystallize in 85% ethylene glycol dimethyl ether solution, dehydrate with dehydrating agent anhydrous m...
Embodiment 2
[0020] The synthetic method of pharmaceutical intermediate 1,4,9(11)-pregnatriene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate comprises the following steps:
[0021] A: Add 3mol 2-hydroxyl-4,9(11)-pregnadiene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate in the reaction vessel, 900ml quality For a propionitrile solution with a fraction of 33.5%, raise the solution temperature to 53.5°C, add 3.5mol potassium peroxodisulfate, 1.2L oxalic acid solution with a mass fraction of 23%, and react for 50 minutes;
[0022] B: raise the temperature of the solution to 63°C, add 3.5mol of chloroauric acid powder, 800ml of sodium sulfate solution with a mass fraction of 18.5%, continue the reaction for 2.5h, adjust the pH to 6.25, let stand for 40min, and use a mass fraction of 12.5% Wash 4 times with sodium chloride solution, wash 3 times with 73% ethylbenzene solution, recrystallize in 88.5% ethylene glycol dimethyl ether solution, dehydrate with dehydrating agent anhydrous magnesium...
Embodiment 3
[0024] The synthetic method of pharmaceutical intermediate 1,4,9(11)-pregnatriene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate comprises the following steps:
[0025] A: Add 3mol 2-hydroxyl-4,9(11)-pregnadiene-16α-methyl-17α,21-diol-3,20-diketone-21-acetate in the reaction vessel, 900ml quality For a propionitrile solution with a fraction of 37%, raise the temperature of the solution to 57°C, add 4 mol of potassium peroxodisulfate, 1.2L of an oxalic acid solution with a mass fraction of 26%, and react for 60 minutes;
[0026] B: Raise the temperature of the solution to 66°C, add 4mol of chloroauric acid powder, 800ml of sodium sulfate solution with a mass fraction of 22%, continue the reaction for 3h, adjust the pH to 6.5, let it stand for 50min, and use 15% of the mass fraction to chlorinate Wash 5 times with sodium solution, wash 4 times with ethylbenzene solution with mass fraction of 76%, recrystallize in ethylene glycol dimethyl ether solution with mass fraction of 92%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com