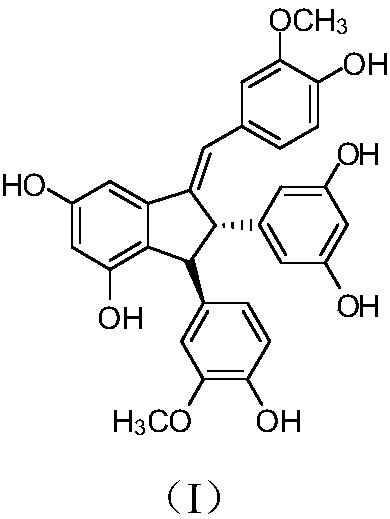
Application of toluylene dimer in preparing medicine for treating liver related diseases
A stilbene dimer and drug technology, applied in the direction of drug combination, resistance to vector-borne diseases, antiviral agents, etc., can solve the problems that the activity has not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
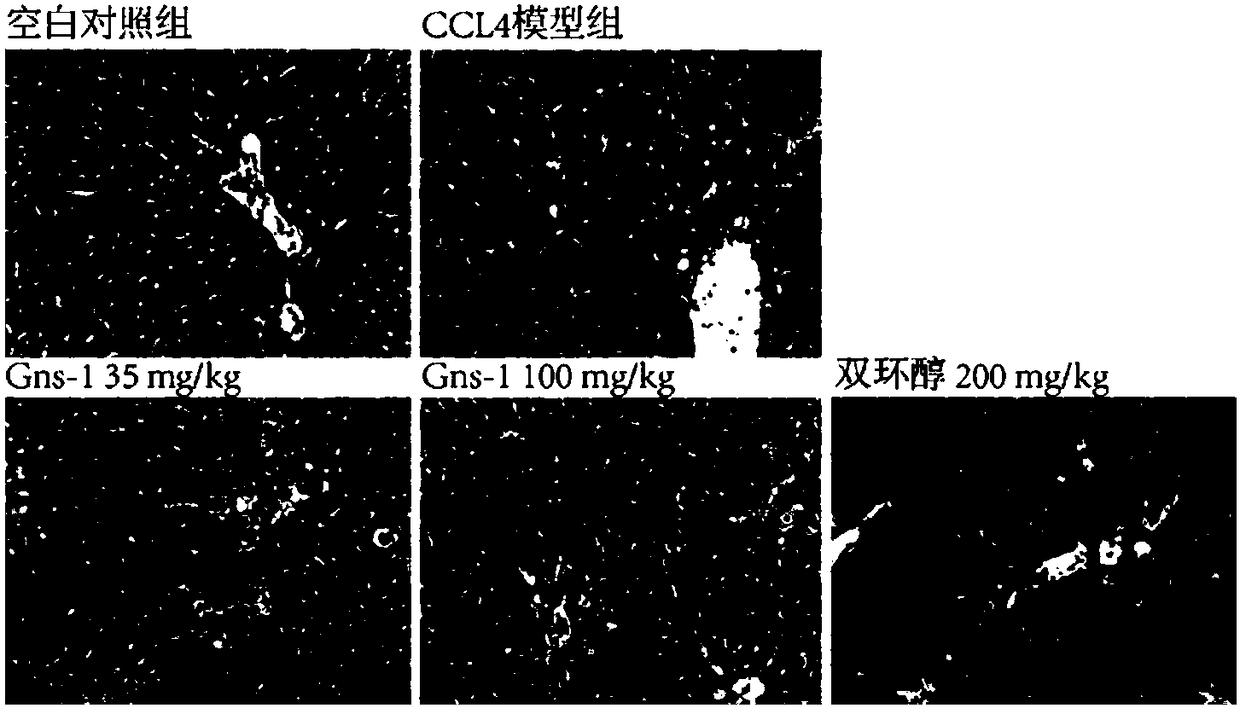
[0024] Example 1 Detection of Gns-1 using APAP-induced liver cell injury model in vitro
[0025] 1. Experimental method: MTT method is adopted. HepG2 cells are human liver cancer cells, which retain most of the characteristics of normal human liver cells, and are often used in the study of liver cell injury. HepG2 cells were inoculated in 96-well cell culture plates, and after 24 hours of culture, non-toxic concentrations of Gns-1 and paracetamol (APAP, final concentration 8mM) were added, and the positive drug bicyclol control group, solvent control group and model group were set at the same time. Continue to act on the cells for 48h. Discard the culture medium, add 100 μL of MTT (0.5 mg / mL) solution to each well, continue to incubate for 4 h, discard the MTT solution, add 150 μL of DMSO to each well, shake with a mixing oscillator, and measure the absorbance value at a wavelength of 570 nm on a microplate reader. Cell survival rate (%)=100×average OD of administration grou...
Embodiment 2
[0030] Example 2 Detection of Gns-1 by triptolide-induced liver cell injury model in vitro
[0031] 1. Experimental method: MTT method is adopted. HepG2 cells were seeded in 96-well cell culture plates, and after 24 hours of culture, non-toxic concentrations of Gns-1 and triptolide (TPL, final concentration 180nM) were added, and the positive drug bicyclol control group, solvent control group and model were set at the same time. Group. Continue to act on the cells for 48h. Discard the culture medium, add 100 μL of MTT (0.5 mg / mL) solution to each well, continue to incubate for 4 h, discard the MTT solution, add 150 μL of DMSO to each well, shake with a mixing oscillator, and measure the absorbance value at a wavelength of 570 nm on a microplate reader. Cell survival rate (%)=100×average OD of administration group / average OD of blank control group. Experiments were repeated 3-4 times.
[0032]2. Experimental results: The results are shown in Table 2. TPL 180nM acted on HepG...
Embodiment 3
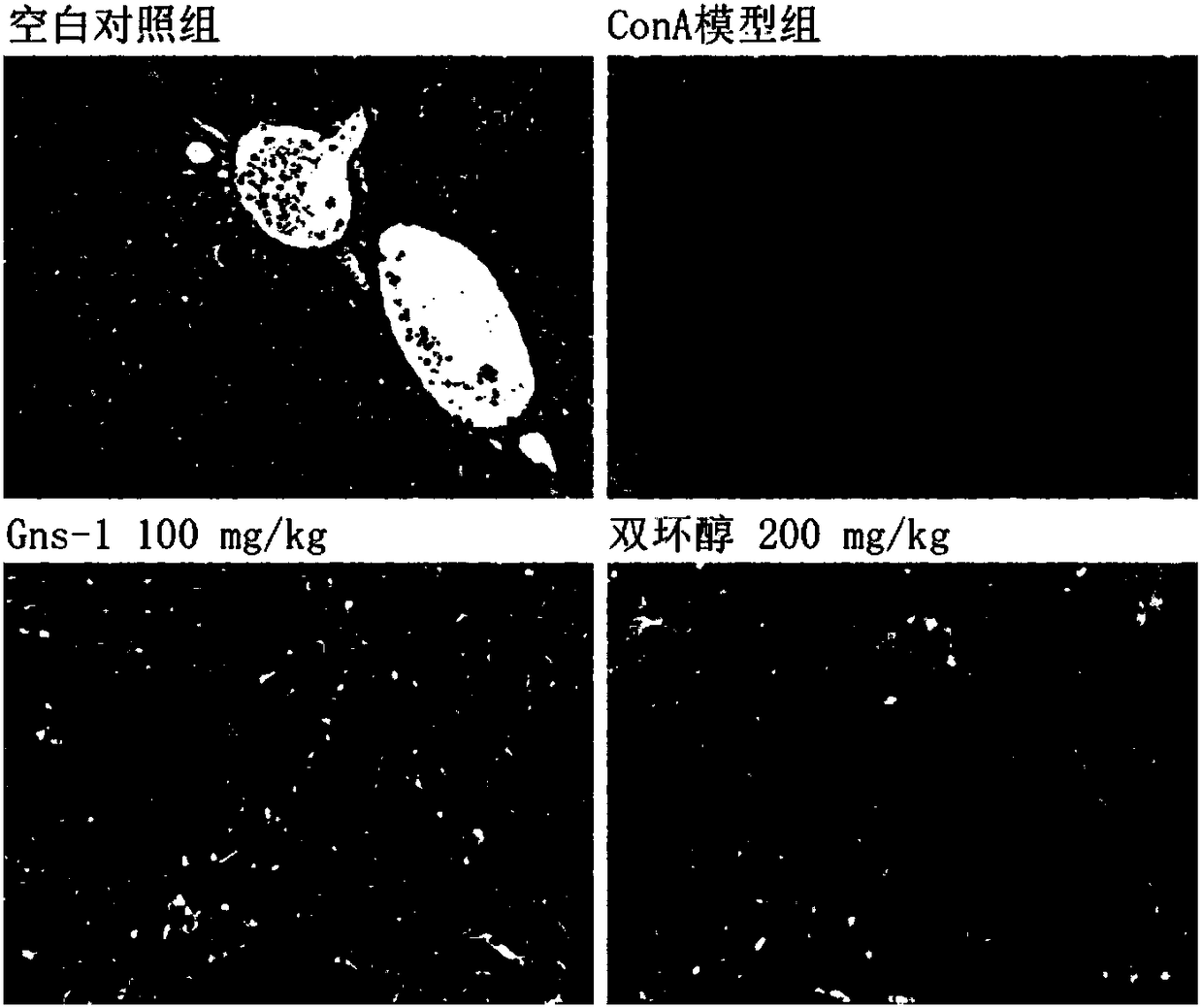
[0037] Example 3 Using concanavalin A (ConA)-induced acute immune liver injury model to detect Gns-1
[0038] 1. Experimental methods and detection indicators
[0039] 1.1 Establishment of ConA-induced acute immune liver injury model in mice and administration method
[0040] SPF male ICR mice (20-22g) were randomly divided into 4 groups after adapting to the environment, blank control group, ConA model group, Gns-1 100mg / kg group by intragastric administration, and positive control drug bicyclol intragastric administration 200mg / kg group. The Gns-1 administration group started administration 3 days before modeling, and administered once a day for a total of 3 days. The bicyclol group administered once in the afternoon of the day before modeling, and in the morning and afternoon of the same day. , Blank control group and model group animals were given the same amount of 0.5% CMC-Na solution. Two hours after the last administration, except the blank control group, the mice i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com