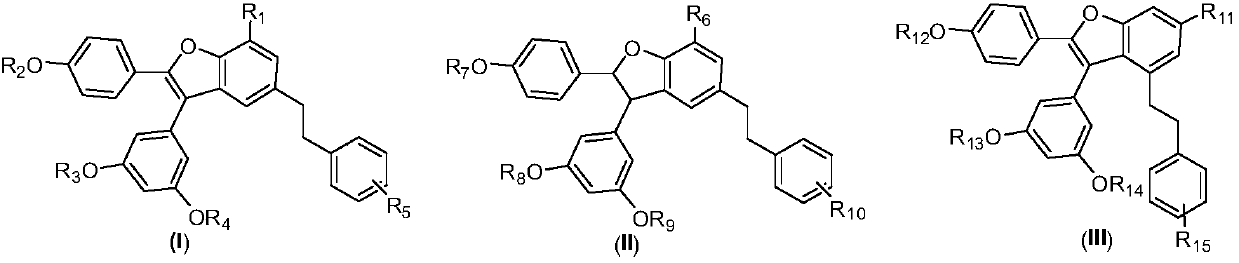
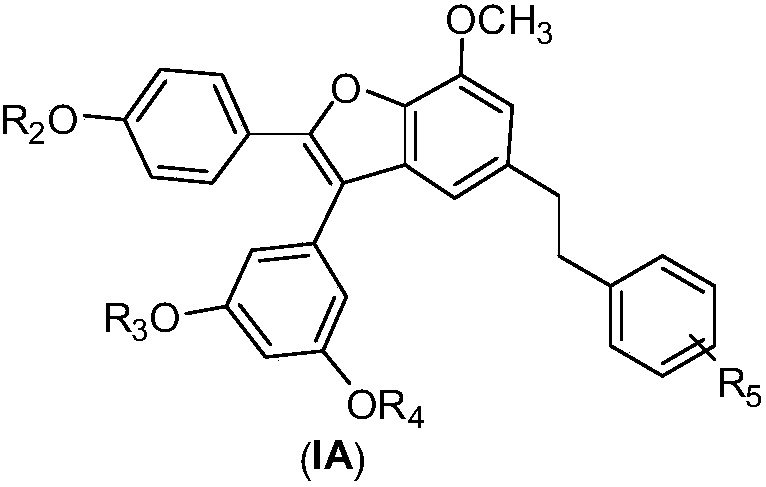
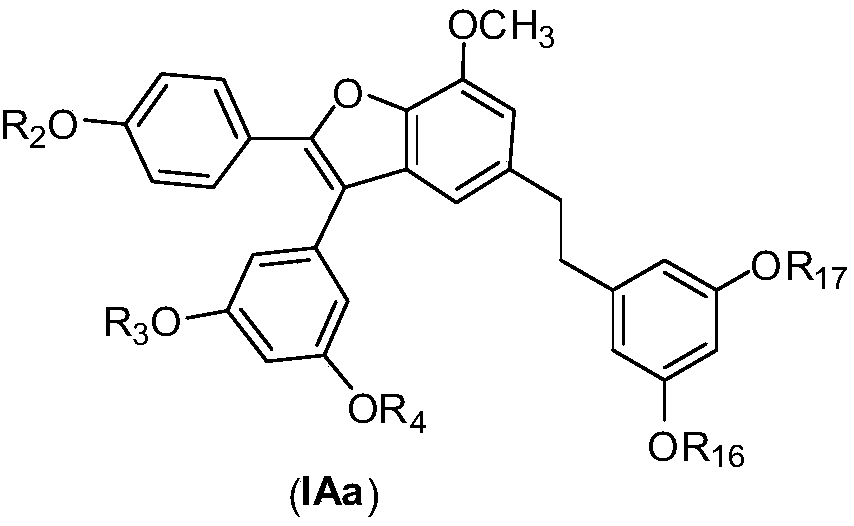
Applications of Amurensin H derivatives in preparation of drugs for treating liver related diseases
A technology of glupapentin and its derivatives, which is applied in the field of biomedicine and can solve the problems of few research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0115] In order to further illustrate the present invention, a series of examples are given below (the compound code number is corresponding to the compound code number in the examples), these examples are completely illustrative, and they are only used to specifically describe the present invention, and should not be interpreted as Invention Limitations.
[0116] The synthetic route of intermediate compound 1c in the embodiment:
[0117]
[0118] The synthetic route of intermediate compound 2b, 3b, 2c and 3c in the embodiment:
[0119]
Embodiment 1
[0121] 5-[6-Acetoxy-2-[4-acetoxyphenyl]-4-[2-(4-acetoxyphenyl)ethyl]-3-benzofuranyl]-1,3 -Diphenol-1,3-diacetate (1)
[0122] The synthetic route of compound 1:
[0123]
[0124] 100 mg of compound 1c was dissolved in 6 ml of ethyl acetate, 7.11 mg of Pd / C (10%) was added, reacted under pressure for 1 h under a hydrogen atmosphere, filtered to remove Pd / C, and the filtrate was concentrated under reduced pressure to obtain 93.8 mg (0.140 mmol) of white solid compound 1 ), yield 93.5%, m.p.79-81°C.
[0125] Compound 1: UVλ max (MeOH,logε):307.4(4.43)nm; 1 H NMR (CD 3 COCD 3 ,500MHz)δ:7.55(2H,d,J=8.5Hz,H-2a,6a),7.31(1H,d,J=1.5Hz,H-14b),7.26(2H,d,J=2.0Hz, H-10a, 14a), 7.12 (1H, t, J = 2.0Hz, H-12a), 7.09 (2H, d, J = 8.5Hz, H-3a, 5a), 6.89 (1H, J = 1.5Hz, H-12b),6.87-6.88(4H,m,H-2b,6b,3b,5b),2.79-2.83(2H,m,H 2 -7b),2.61-2.65(2H,m,H 2-8b),2.29(3H,s,OCOCH 3 ),2.24(6H,s,OCOCH 3 ),2.21(3H,s,OCOCH 3 ); 13 C NMR (CD 3 COCD 3 ,125MHz) δ: 169.8 (O C OCH 3 ), 169.6 (O C...
Embodiment 2
[0127] 5-[6-Hydroxy-2-(4-hydroxyphenyl)-4-[2-(4-hydroxyphenyl)ethyl]-3-benzofuryl]-1,3-benzenediol (2 )
[0128] The synthetic route of compound 2:
[0129]
[0130] 100mg of compound 1 (0.154mmol) was dissolved in 3ml of dichloromethane, diluted with 3ml of methanol, and then added with 1855mg of NH 4 OAc (24.096 mmol), stirred at room temperature for 4d. After the reaction was complete, water and ethyl acetate were added for extraction, and the organic phases were combined and concentrated to dryness under reduced pressure to obtain 62 mg (0.137 mmol) of compound 2 as a yellow solid with a yield of 90.4%, m.p.240-242°C.
[0131] Compound 2: UVλ max (MeOH,logε):284.6(4.34),319.2(4.54)nm; 1 H NMR (CD 3 COCD 3 ,500MHz)δ:8.18-8.62(br s,H-OH),7.41(2H,d,J=8.5Hz,H-2a,6a),6.85 7.31(1H,d,J=2.0Hz,H-14b ),6.78(2H,d,J=8.5Hz,H-2b,6b),6.77(2H,d,J=8.5Hz,H-3a,5a),6.64(2H,d,J=8.5Hz,H -3b,5b),6.62(1H,J=2.0Hz,H-12b),6.51(1H,t,J=1.5Hz,H-12a),6.49(2H,d,J=1.5Hz,H-10a ,14a),2.68-2.71(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com