Application of metallide/palladium compound catalytic reduction system in reaction of removing allyl groups and deuteration reaction
A palladium compound, metallization technology, applied in the direction of organic compound/hydride/coordination complex catalyst, cyanide reaction preparation, preparation of organic compounds, etc., to achieve the effect of wide substrate range, small molecular weight and easy availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
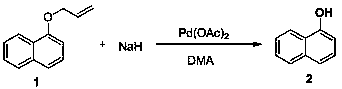
[0031]
[0032] Under nitrogen protection, palladium acetate (0.018 mmol, 6 mol%) and sodium hydride (60% in oil, 0.51 mmol, 1.7 equiv) were suspended in DMA (1.0 mL), stirred at 25°C for 5 minutes, and compound 1 (0.3 mmol) was added solution in DMA (0.5mL), then reacted at 25°C for 4 hours, added ice water to stop the reaction, adjusted the pH value to 3.5 with dilute hydrochloric acid, extracted with ethyl acetate, combined the extracts, dried with sodium sulfate, and rotary evaporated Drying and purification by column chromatography gave product 2 with a yield of 99%. 1 H NMR (400 MHz, DMSO-d 6 ): δ 10.10 (br, 1H), 8.13 (d, J = 7.9 Hz, 1H), 7.80 (d, J =7.7 Hz, 1H), 7.51-7.37 (m, 2H), 7.31 (q, J = 8.0 Hz, 2H), 6.87 (d, J = 6.8Hz, 1H); 13 C NMR (151 MHz, DMSO-d 6 ): δ 153.12, 134.38, 127.34, 126.39, 126.04, 124.53, 124.50, 121.94, 118.29, 108.00. LR-MS (ESI): m / z 145.1 [M+H] + .
Embodiment 2
[0034]
[0035]Under nitrogen protection, palladium chloride (0.006 mmol, 2 mol%) and lithium hydride (0.9 mmol, 3 equiv) were suspended in THF (1.0 mL), stirred at 25°C for 5 minutes, compound 1 (0.3 mmol) was added in THF (0.5 mL), then reacted at 80°C for 1 hour, added ice water to stop the reaction, adjusted the pH value to 3.5 with dilute hydrochloric acid, extracted with ethyl acetate, combined the extracts, dried with sodium sulfate, evaporated to dryness, and column layer Purified by analysis to obtain product 2 with a yield of 92%.
Embodiment 3
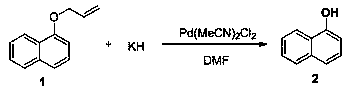
[0037]
[0038] Under nitrogen protection, Pd(MeCN) 2 Cl 2 (0.003 mmol, 1 mol%) and potassium hydride (0.3 mmol, 1 equiv) were suspended in DMF (1.0 mL), stirred at 25°C for 5 minutes, a solution of compound 1 (0.3 mmol) in DMF (0.5 mL) was added, and then in React at -50°C for 36 hours, add ice water to stop the reaction, adjust the pH value to 3.5 with dilute hydrochloric acid, extract with ethyl acetate, combine the extracts, dry with sodium sulfate, evaporate to dryness by rotary evaporation, and purify by column chromatography to obtain the product 2 , yield 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com