Preparation method of low residual alkali Li(NiCoAl)O2 cathode material
A technology of nickel-cobalt-lithium-aluminate and cathode materials, applied in battery electrodes, electrical components, circuits, etc., can solve problems such as battery explosion, reduced battery capacity, cycle life, and battery volume expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] One aspect of the present invention provides a method for preparing a nickel-cobalt lithium aluminate positive electrode material, wherein the method comprises the following steps:
[0044] Step 1. Mix the nickel-cobalt-aluminum hydroxide precursor with the lithium source, and perform pre-sintering.
[0045] According to a preferred embodiment of the present invention, in step 1, the nickel cobalt aluminum hydroxide precursor has the formula Ni 1-x-y co x Al y (OH) 2 Indicates that 0
[0046] According to a preferred embodiment of the present invention, in step 1, the lithium source is selected from lithium carbonate, lithium hydroxide, lithium nitrate, lithium oxalate, lithium fluoride, lithium bromide, lithium chloride, lithium acetate, lithium oxide, One or more of lithium dihydrogen phosphate and lithium phosphate.
[0047] In a further preferred embodiment, the lithium source is selected from one or more of lithium carbonate, lithium hydroxide,...
Embodiment approach
[0052] According to a preferred embodiment of the present invention, the mixing is by ball milling.
[0053] In a further preferred embodiment, the mixing adopts inclined ball milling.
[0054] Wherein, the purpose of mixing is to uniformly mix the lithium source and the nickel-cobalt-aluminum hydroxide precursor.
[0055] According to a preferred embodiment of the present invention, the mixing frequency of the ball mill is 8-14 Hz.
[0056] In a further preferred embodiment, the mixing frequency of the ball mill is 8-12 Hz.
[0057] In a further preferred embodiment, the frequency of the ball mill mixing is 8-10 Hz.
[0058] According to a preferred embodiment of the present invention, the mixing time of the ball mill is 2-10 hours.
[0059]In a further preferred embodiment, the time for ball milling and mixing is 2 to 6 hours.
[0060] In a further preferred embodiment, the ball milling time is 2-4 hours.
[0061] According to a preferred embodiment of the present inven...
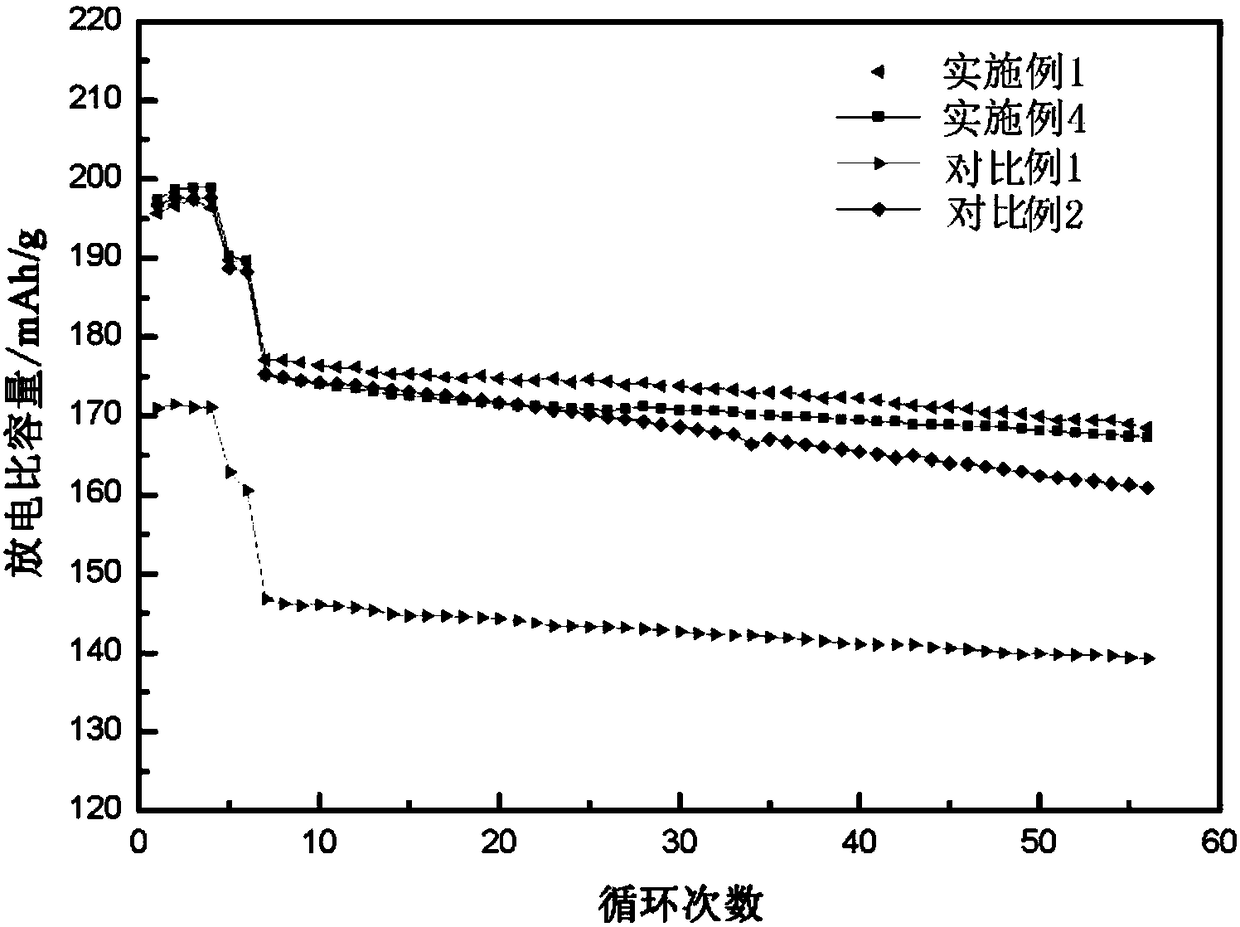
Embodiment 1
[0108] Mix 50.00g of nickel-cobalt-aluminum hydroxide precursor with 23.20g of lithium carbonate, wherein the molar ratio of lithium source to NCA is 1.05, ball mill at 8Hz for 10h, and pre-sinter at 500°C for 6h, where the heating rate is 2°C / min, then cool down naturally;
[0109] The material obtained in step 1 is crushed, passed through a 300-mesh sieve, sieved 4 times, and then sintered for the second time. The sintering process is 5°C / min to 500°C, then 2°C / min to 760°C, and heat preservation for 10h After cooling with the furnace, the matrix material is obtained;
[0110] The matrix material obtained in step 2 was broken, and 2.93g of nano-cobalt hydroxide was added, passed through a 300-mesh sieve, and then sintered three times. ℃, keep warm for 6 hours, and then cool with the furnace to obtain nickel-cobalt-lithium-aluminate cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com