Anti-tumor targeting drug delivery system as well as preparation method and application thereof
A targeted drug delivery and anti-tumor technology, applied in anti-tumor drugs, pharmaceutical formulations, drug combinations, etc., can solve the problem of long experimental period of exosome method, poor cell targeting ability of nucleic acid drugs, poor endocytosis and escape of endosomes, etc. To achieve the effect of inhibiting breast cancer tumor proliferation, inhibiting tumor proliferation, and weakening the inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Collection of Exosomes
[0049] The culture supernatant of immature dendritic cells (iDC) derived from cultured mice was collected and centrifuged at 300×g for 10 min. The supernatant was concentrated by the filter tube (5000×g, 30min) to obtain a concentrated solution, which was then diluted to the original volume with PBS, and concentrated again by centrifugation at 5000×g for 30min in a 100kDa ultrafiltration tube. Then add exosome extract according to the volume ratio of concentrate: exosome extract reagent = 2:1, mix thoroughly, and incubate overnight at 4°C. Finally, centrifuge at 12000×g for 2 hours, remove the supernatant, collect exosome, and resuspend with sterilized PBS. After testing the protein concentration with a Nandrop 2000 spectrophotometer, store at -20°C, and store at 4°C within one month.
[0050] With about 10 μg of purified exosome, add an equal volume of 4% glutaraldehyde, and fix at room temperature for 30 min. Then put the sample ...
Embodiment 2
[0052] Example 2: Preparation of AS1411-exosome
[0053] The specific steps are:
[0054](1) Stock solution A and B configuration: Weigh 3 mg of cholesterol-labeled polypeptide (cholesterol-IIASTIGGIFGSSTTQSGGGG) and dissolve it in 1.5 mL of sterile water to prepare 2 mg / mL stock solution A; 5OD labeled CY3 nucleic acid aptamer AS1411 (5′-TTGGTGGTGGTGGTTGTGGTGGTGGT GG-CY3-3′,) was dissolved in 500 μL sterile water to prepare stock solution B;
[0055] (2) Take three glass bottles and add 500 μL of stock solution A to each bottle; then add equimolar EDC and NHS aqueous solutions respectively, and stir at room temperature for 2 hours to activate the polypeptide;
[0056] (3) Add 500 μL of stock solution B to the solution in step 2 above, and stir at room temperature for 4 days.
[0057] (4) After the reaction is finished, dialyze overnight with a dialysis bag with a molecular weight of 3 kDa to obtain a polypeptide modified by the nucleic acid aptamer.
[0058] (5) 150 μg of ...
Embodiment 3
[0061] Example 3: Preparation of AS1411-exosome-let-7
[0062] Mix 150 μg AS1411-exosome and 150 μg miRNA let-7 with OPTI-MEM serum-free medium, add to a 0.4 cm electric shock cup, ice bath, and then use Eppendorf’s Eppendorf Co. Multiporator click to load.
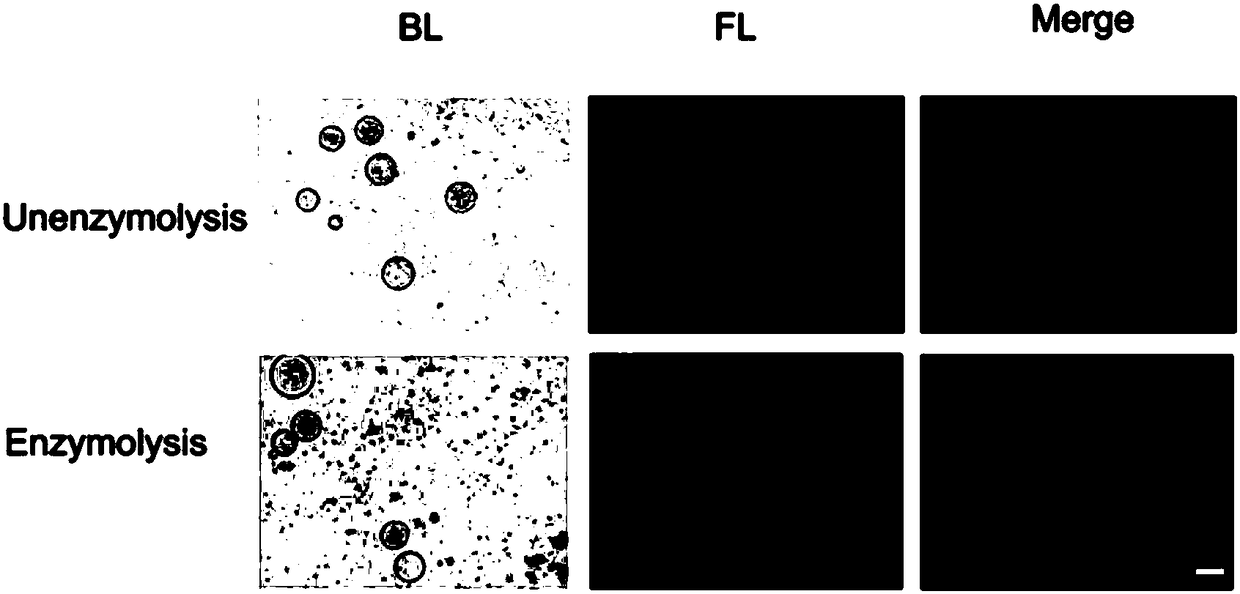
[0063] In order to further judge whether the loading was successful or not, Cy3-labeled let-7 was used for loading and fluorescence microscopy was used to analyze the fluorescence intensity of AS1411-exosome-let-7-Cy3 adsorbed by magnetic beads. From image 3 It can be seen that the magnetic beads have a strong fluorescent signal after adsorbing AS1411-exosome-let-7-Cy3, indicating that let-7-Cy3 was successfully loaded into the AS1411-exosome by electric shock, and endowed AS1411-exosome with a strong fluorescent signal ( Scale bar is 100 μm).
[0064] The loaded AS1411-exosome-let-7 was observed under a transmission electron microscope to determine the effect of electric shock on the morphology of the complex. resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com