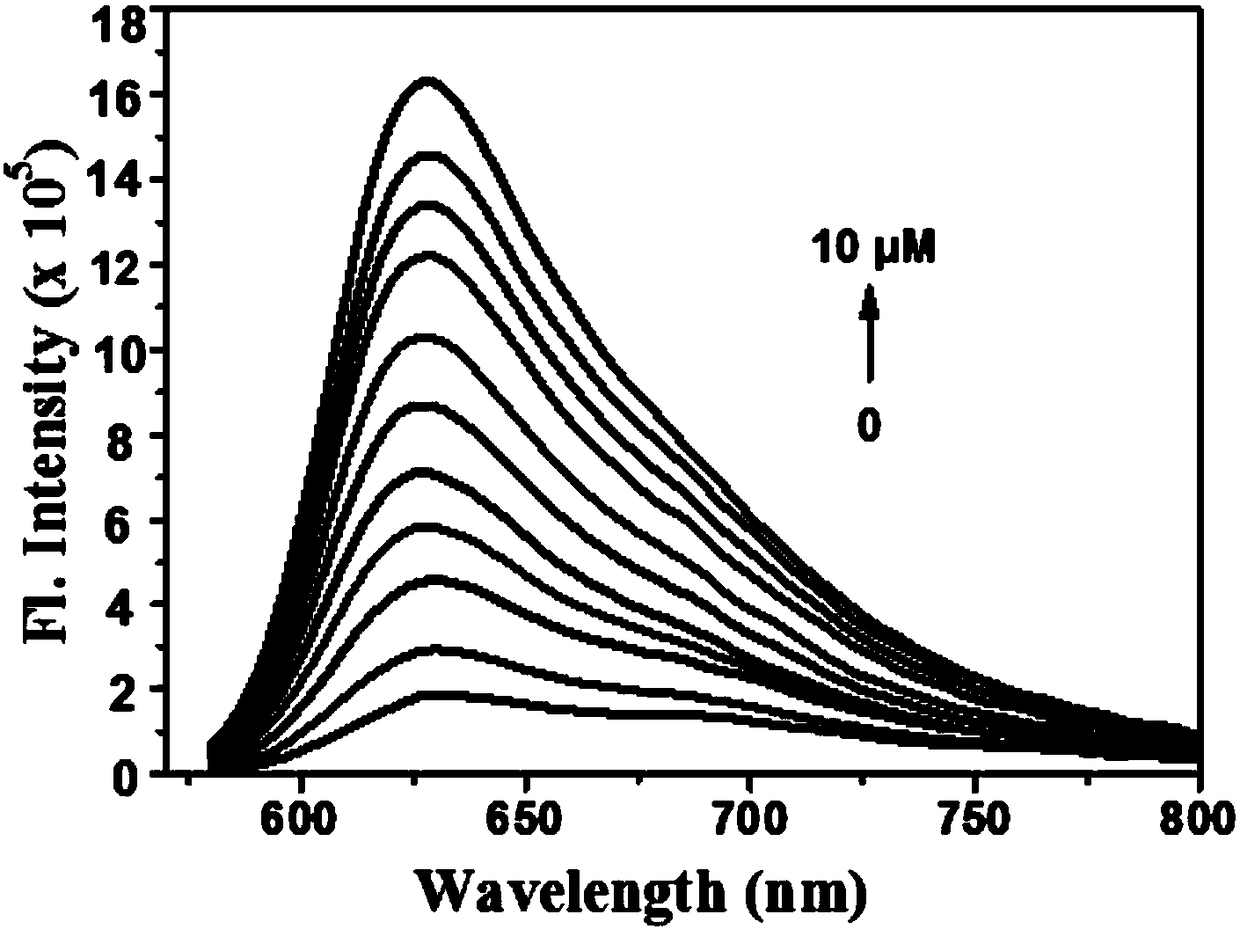
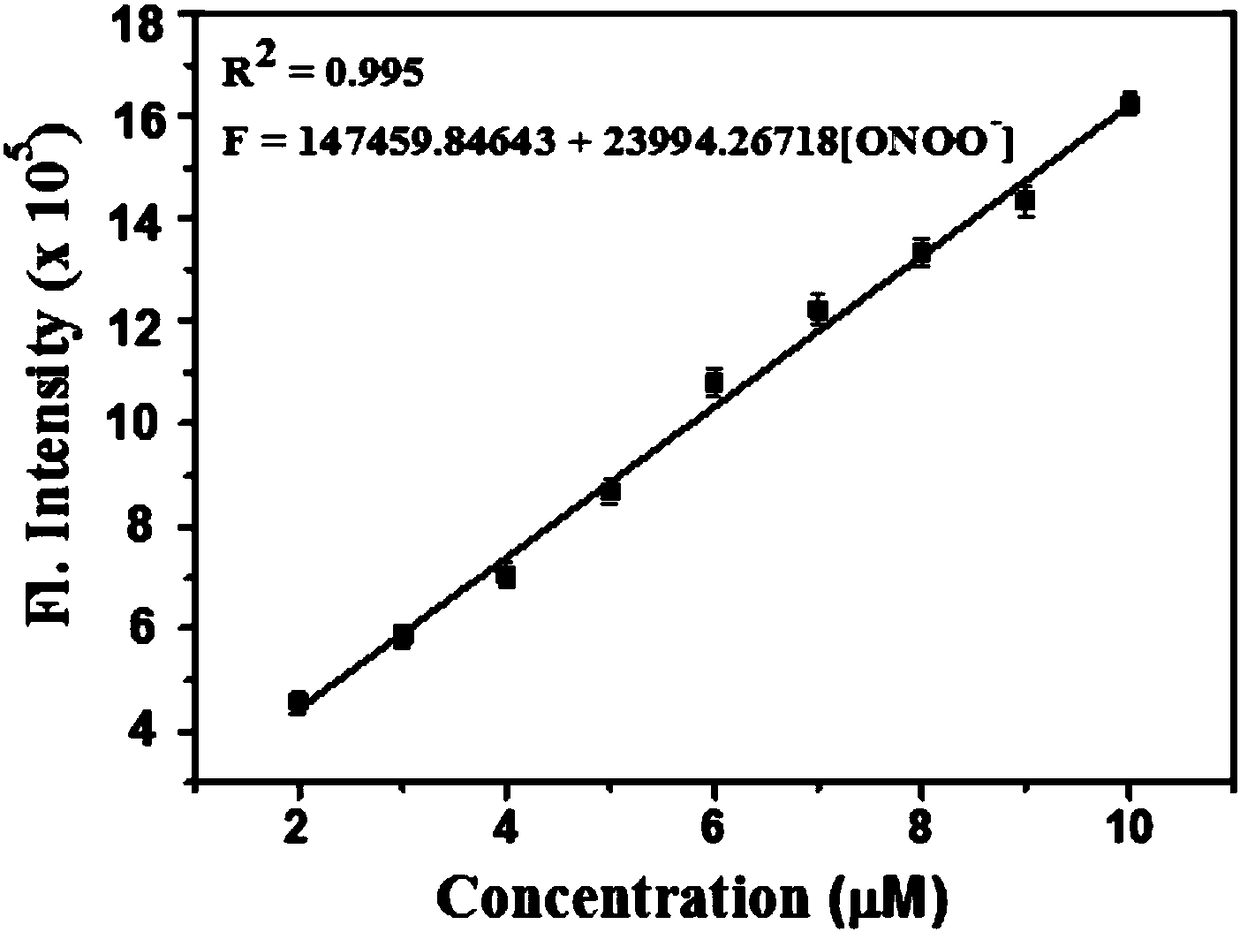
Two-photon fluorescence probe for detecting peroxynitrite as well as preparation method and application of two-photon fluorescence probe
A technology of peroxynitrite and two-photon fluorescence, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, which can solve the problem of fewer fluorescent probes and reduce self-absorption and yield High, highly sensitive and selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0046] Example: Synthesis of fluorescent probe
[0047] Under the protection of argon, the compound TPNIR-NH 2 (160 mg, 0.5 mmol) was dissolved in 10 mL of anhydrous dichloromethane, and triethylamine (58 μL, 2.0 mmol) was added, and stirred at room temperature for 5.0 min. Subsequently, compound 1 (1.06 g, 5.0 mmol) was dissolved in 5.0 mL of dichloromethane, added dropwise to the above mixture, and stirring was continued for 1.5 h at room temperature. After the completion of the reaction, the solvent was concentrated and spin-dried, and the solid was separated by column chromatography (eluent: dichloromethane: methanol 200:1, V / V) to obtain 174 mg of purple black solid (yield 70%).
[0048] Nuclear magnetic and mass spectrometry characterization:
[0049] 1 H NMR(400MHz, DMSO-d 6 ): δ11.42(s,1H),8.69(s,1H), 8.40(d,J=8.9Hz,2H), 8.34(d,J=8.9Hz,2H), 8.25(d,J=9.4Hz ,1H),7.95(s,2H),7.93(s,1H),7.47(dd,J=9.4,2.2Hz,1H),7.30(d,J=1.9Hz,1H),3.70(q,J= 7.0Hz, 4H), 3.08 (br s, 4H), 1.26 (t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com