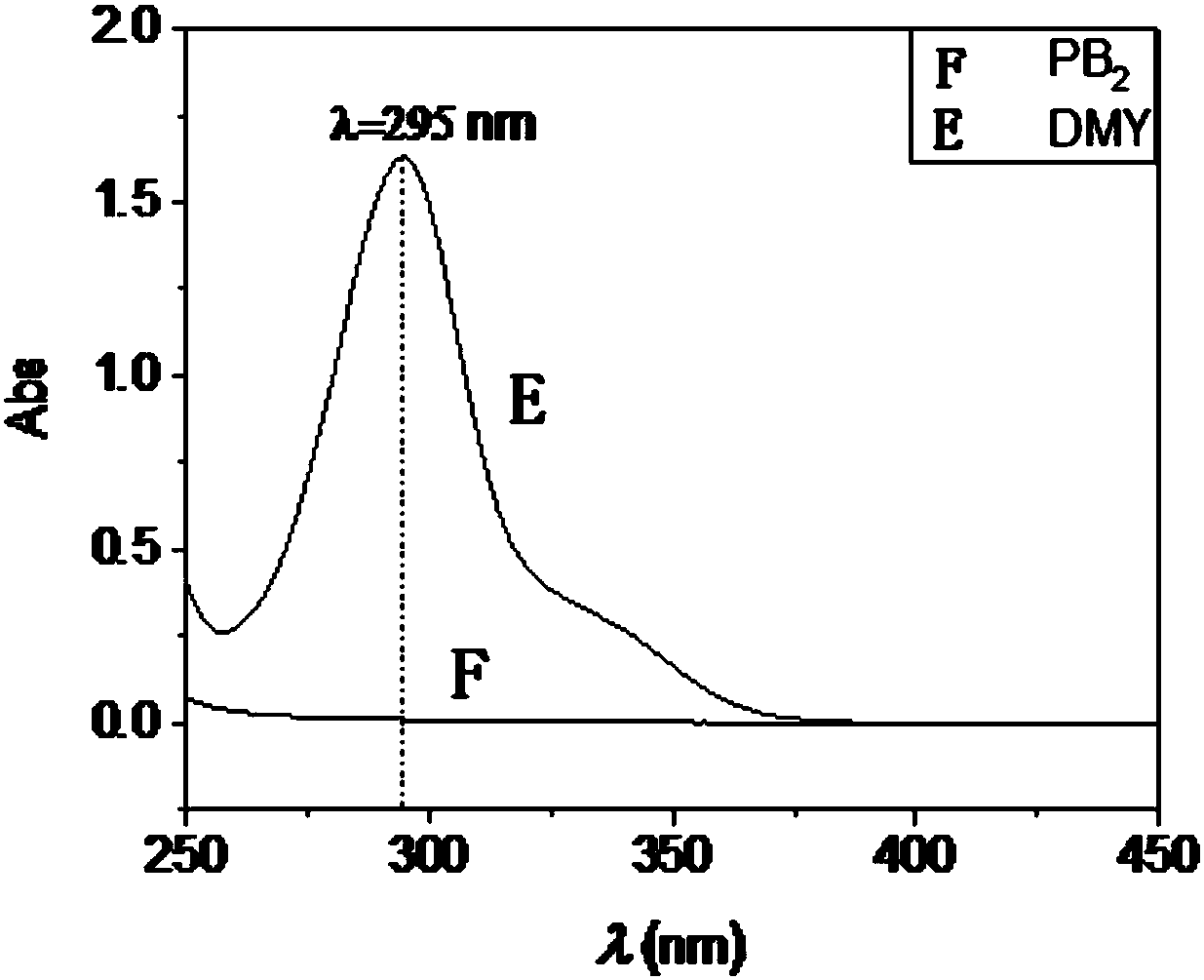
Dihydromyricetin medicament taking biocompatible micro-emulsion as carrier, preparation method and application
A dihydromyricetin, biocompatible technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., can solve problems such as the limitation of the ability to form aggregates, and achieve good compatibility performance , the effect of small viscoelasticity and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
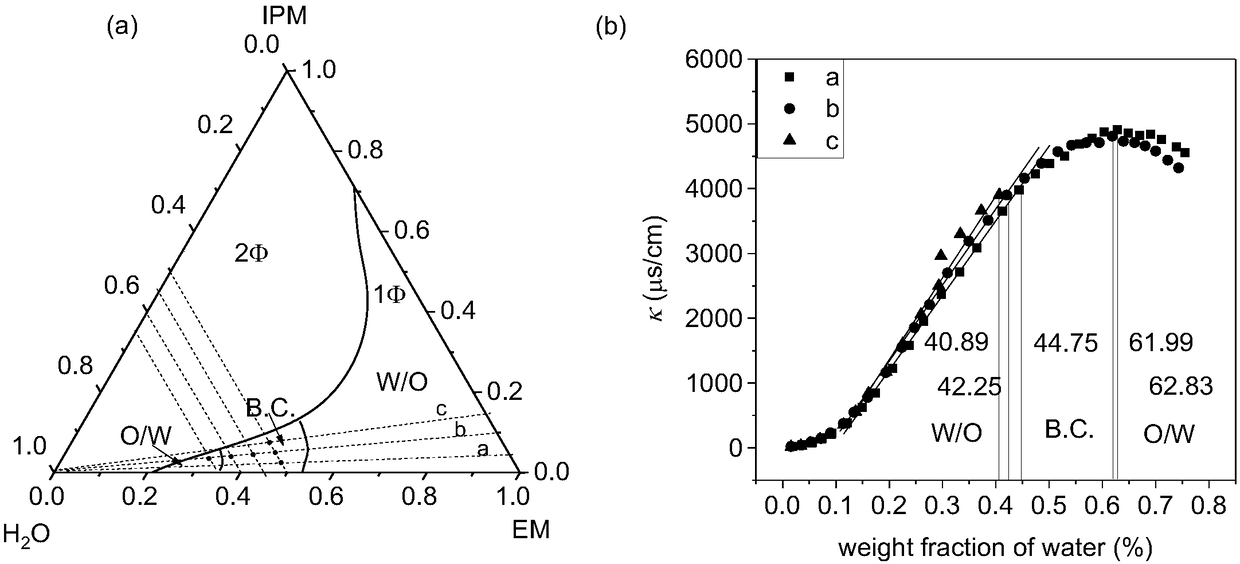
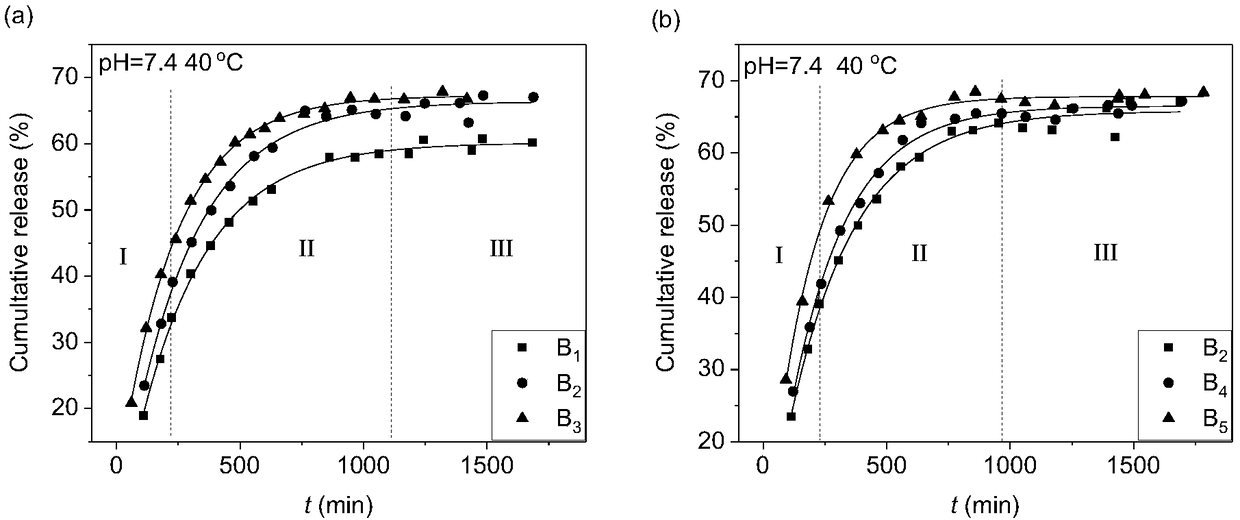
[0051] 1.995g sucrose stearate, 0.855g sodium deoxycholate, 1.9g isopropanol and 0.25g isopropyl myristate are placed in a stoppered colorimetric tube, and they are mixed evenly with a vortex mixer, and Stir fully in a constant temperature water bath at 50-60°C to dissolve it. Then, 5 g of twice distilled water was added dropwise to the colorimetric tube, placed in a water bath, stirred and mixed, and finally left to stand in a constant temperature water bath at 40°C to achieve phase equilibrium, and the microemulsion obtained was the drug-loaded microemulsion, denoted as B 1 .
Embodiment 2
[0053] Place 1.89g sucrose stearate, 0.81g sodium deoxycholate, 1.8g isopropanol and 0.5g isopropyl myristate in a colorimetric tube with a stopper, and mix it evenly with a vortex mixer, Stir fully in a constant temperature water bath at 50-60°C to dissolve it. Then, 5 g of twice distilled water was added dropwise to the colorimetric tube, placed in a water bath, stirred and mixed, and finally left to stand in a constant temperature water bath at 40°C to achieve phase equilibrium, and the microemulsion obtained was the drug-loaded microemulsion, denoted as B 2 .
Embodiment 3
[0055] 1.785g sucrose stearate, 0.765g sodium deoxycholate, 1.7g isopropanol and 0.75g isopropyl myristate are placed in a stoppered colorimetric tube, and they are mixed evenly with a vortex mixer, and Stir fully in a constant temperature water bath at 50-60°C to dissolve it. Then, 5 g of twice distilled water was added dropwise to the colorimetric tube, placed in a water bath, stirred and mixed, and finally left to stand in a constant temperature water bath at 40°C to achieve phase equilibrium, and the microemulsion obtained was the drug-loaded microemulsion, denoted as B 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com