Application of exosomal microRNA molecular markers and kits for diagnosing esophageal cancer
A technology of molecular markers and exosomes, which is applied in the field of medical molecular biology, can solve the problems of insufficient detection specificity of microRNA molecular markers, and achieve the effects of high reverse transcription efficiency, strong primer specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
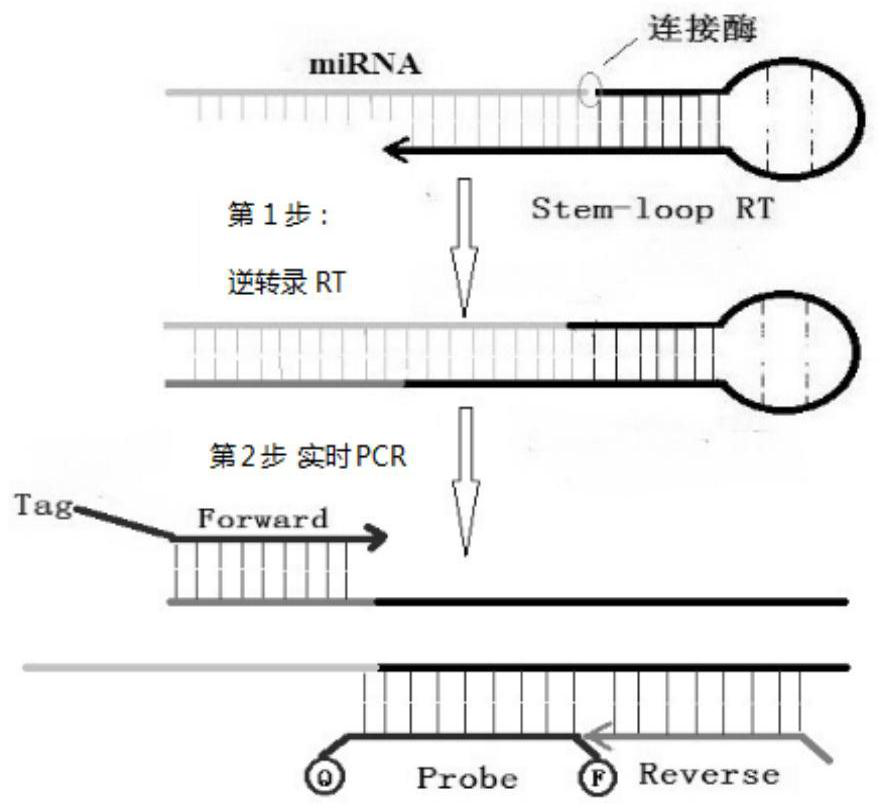
[0069] Example 1 Two-step method detection system kit and use based on PCR platform miRNA
[0070] 1. RNA reverse transcription reaction system:
[0071]Reagents: The reagents used to prepare the reverse transcription reaction system include reverse transcription primers (RT-Primer, synthesized by Shanghai Yingweijieji), miRNA standard powder (synthesized by Shanghai Yingweijieji), T4 DNA ligase (T4 DNA Ligase, Supplier: NEB, product number: M0202S, including 10×T4 DNA Ligase Buffer), RNase inhibitor (RNase inhibitor, supplier: Fermentas, product number: K1622), transcriptase Transcriptase (supplier: Shanghai Yingwei Jieji Biotechnology Co., Ltd., product number: K1622, containing RNase inhibitor, dNTPs, nuclease-free water), T4 polynucleotide kinase (T4 Polynucleotide Kinase, supplier: NEB, product number: M0201S) and nuclease-free pure water (nuclease-free water, supplier: Shanghai Yingwei Jieji Biological Co., Ltd., product number: K1622). The reagents used to prepare the...
Embodiment 2
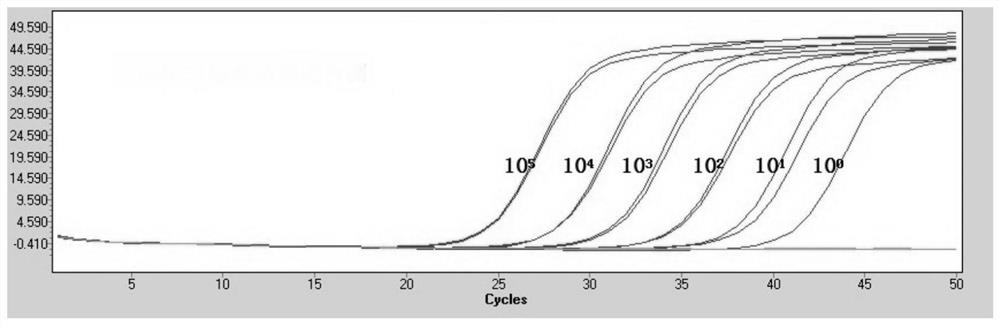
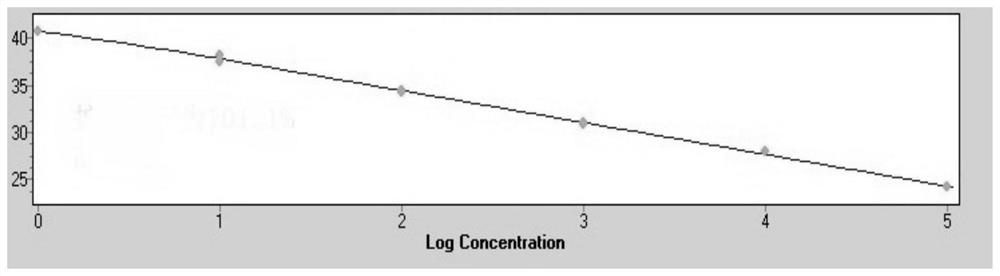
[0096] Example 2 Effect Evaluation of Esophageal Cancer Auxiliary Diagnosis Detection Kit Based on the Two-step Detection System of miRNA
[0097] 1. Sample collection
[0098] Tissue, serum, plasma, and urine samples were collected from a series of people including esophageal cancer (including different stages, different subtypes, different genders, and different age groups), benign lesions of esophageal diseases, and healthy people diagnosed by hospital examination.
[0099] 2. Tissue miRNA extraction and purification
[0100] Using QIAGEN’s commercial product miRNeasy Serum / Plasma Kit (Product No. 217184), extract and purify miRNA in tissue and serum, and use Nano-Drop 2000 to measure RNA nucleic acid quality, record RNA concentration and purity, and normalize tissue miRNA One treatment.
[0101] 3. Extraction and purification of serum exosomal miRNA and plasma exosomal miRNA
[0102] Serum and plasma exosomes were extracted using the commercial product ExoQuickTM kit (p...
Embodiment 3
[0134] Example 3 Exosome miRNA expression level and its difference in body fluid before and after esophageal cancer
[0135] 1. Collect esophageal cancer diagnosed by the hospital (including different stages, different subtypes, different genders and different age groups), and collect preoperative body fluid (serum, plasma, urine) samples from 10 patients with esophageal cancer who have not received any treatment And corresponding postoperative body fluid (serum, plasma, urine) samples. The expression levels of miR-21, miRNA-223, miRNA-25, miRNA-205, miR-203a, miR-375, mi-R-146b-5p, miR-194, and miR-145 were detected.
[0136] 2. The miRNA markers miR-21, miR-223, miR-25, miR-205, miR-375, and miR-203a were detected in clinical samples, and the preoperative body fluids (serum, Plasma, urine) samples and corresponding postoperative body fluid (serum, plasma, urine) samples for Exo-miRNA detection, detection of miRNA markers miR-21, miR-223, miR-25, miR-205, miR- 375, the expr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com