Organic electroluminescence composition and preparation method thereof
A technology of electroluminescent devices and compositions, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of limiting the application space of phosphorescent materials and the high price of phosphorescent materials, and achieve excellent film stability and device efficiency Excellent and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
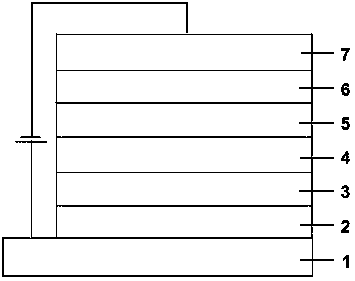
Image
Examples
Embodiment 1
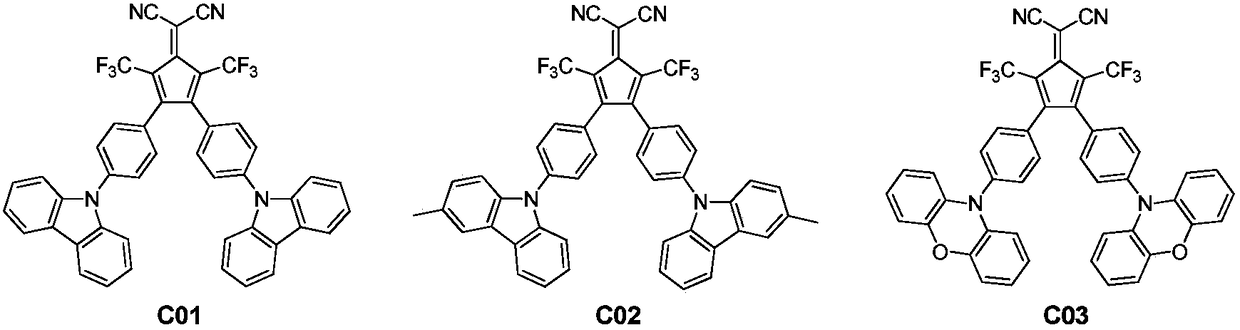
[0034] Embodiment 1: the preparation of compound C01:
[0035]
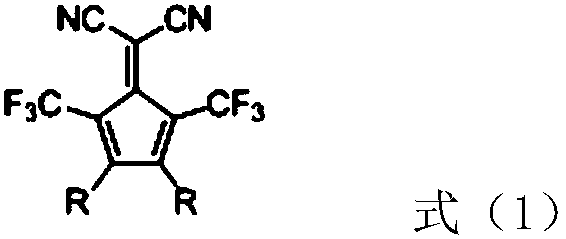
[0036] S1: Preparation of compound 1: prepared by referring to the method described in the literature J.Org.Chem.2010, 75, 7877-7886 (belongs to the prior art, and the specific process will not be described in detail).
[0037] S2: Preparation of compound 2: In a 250mL three-necked flask, add compound 1 (4.2g, 8mmol), carbazole (3.34g, 20mmol), DMF (80g), potassium carbonate (4.14g, 30mmol), cuprous iodide (0.15g, 0.8mmol), 1,10-phenanthroline (0.29g, 1.6mmol), under the protection of nitrogen, heat up to 140°C, keep the temperature for 16h, cool down to 25°C, slowly pour the reaction solution into 300mL Stir in deionized water for 1 hour, filter with suction, rinse with 150 mL of deionized water, collect the filter cake, pass it through a silica gel column for purification, dichloromethane:petroleum ether = 1:1 elution (V / V), and obtain 4.13 g of compound 2 fine product, Yield 74%, structure identified by hi...
Embodiment 2
[0039]Embodiment 2: the preparation of compound C02: its preparation process is basically the same as in Example 1, and its differences are as follows:
[0040]
[0041] Referring to the method described in Example 1, compound C02 was prepared to obtain 2.7 g of the target object, high-resolution mass spectrum, positive ion mode, molecular formula C 48 h 28 f 6 N 4 , theoretical value 774.2218, test value 774.2226, elemental analysis (C 48 h 28 f 6 N 4 ), theoretical value C: 74.41,
[0042] H: 3.64, F: 14.71, N: 7.23, Found C: 74.39, H: 3.67, F: 14.76, N: 7.18.
Embodiment 3
[0043] Embodiment 3: Preparation of compound C03: its preparation process is basically the same as in Example 1, and its differences are as follows:
[0044]
[0045] Referring to the method described in Example 1, compound C03 was prepared to obtain 2.2 g of the target object, high-resolution mass spectrum, positive ion mode, molecular formula C 46 h 24 f 6 N 4 o 2 , theoretical value 778.1803, test value 778.1814, elemental analysis (C 46 h 24 f 6 N 4 o 2 ), theoretical value C: 70.95, H: 3.11, F: 14.64, N: 7.19, O: 4.11, measured value C: 70.92, H: 3.12, F: 14.60, N: 7.25, O: 4.11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Brightness | aaaaa | aaaaa |
| Current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com