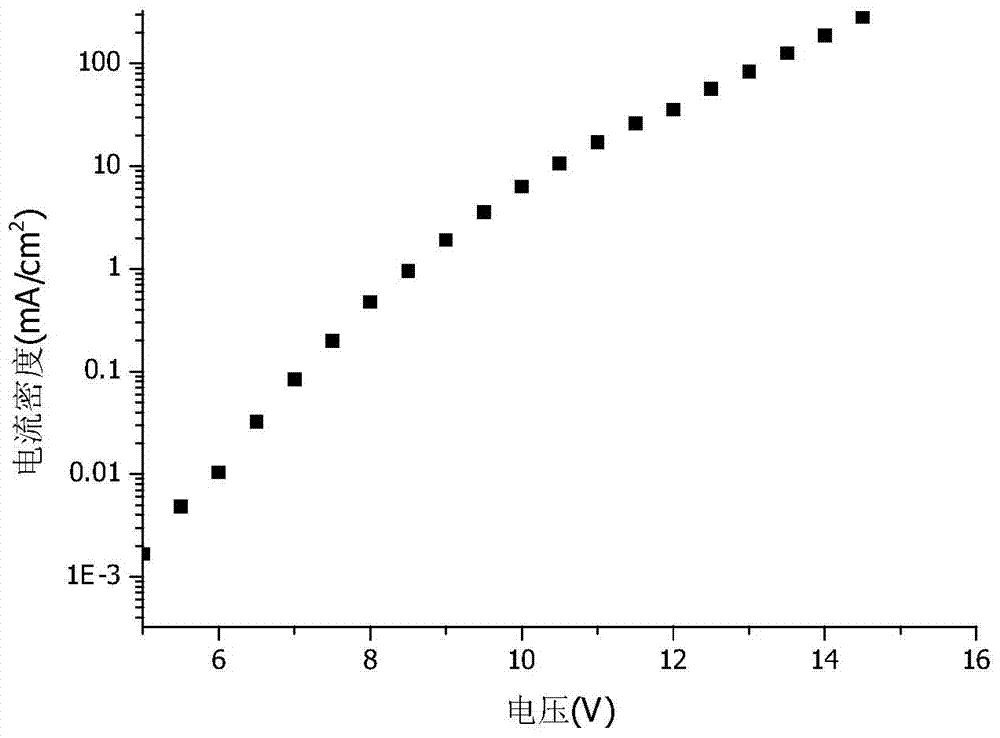
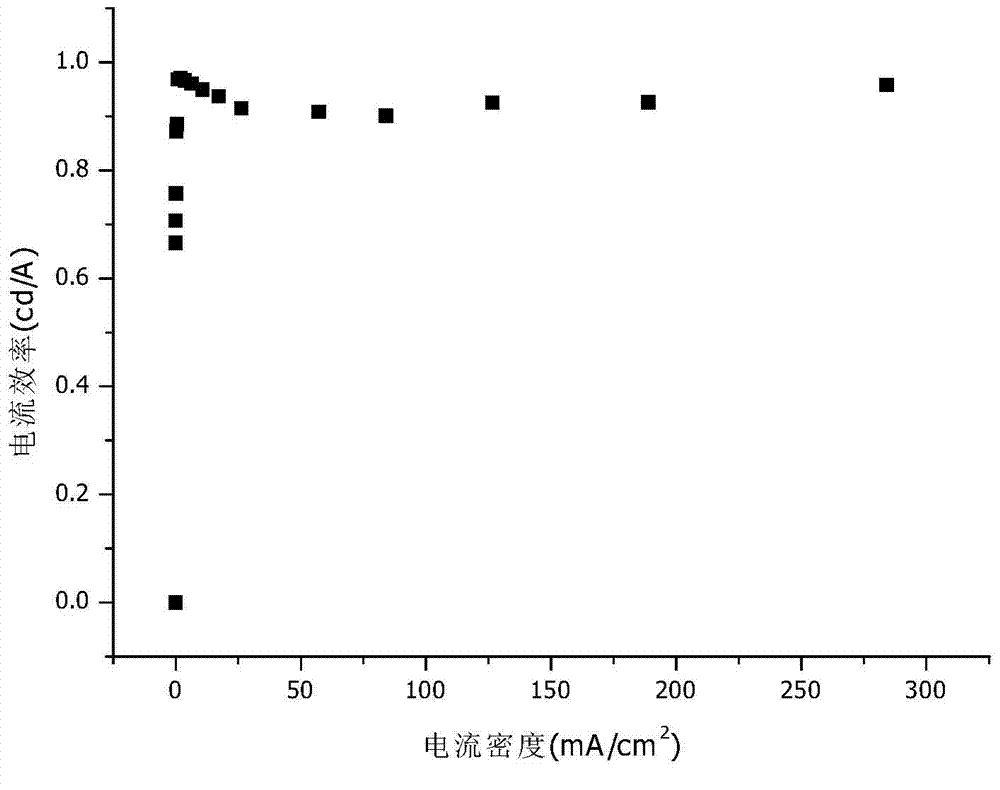
Novel electroluminescent material and application thereof
A technology of electroluminescent materials and electroluminescent devices, applied in the direction of luminescent materials, circuits, electrical components, etc., to achieve the effect of good film stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of 8-(2-methoxy-vinyl)quinoline (compound 1)
[0052] In a 2L three-necked flask, add quinoline-8-carbaldehyde (78.5g, 0.5mol), methoxymethyltriphenylphosphine chloride (205.5g, 0.6mol), toluene (825g), N 2Protection, lower the temperature to the internal temperature <-5°C, dissolve potassium tert-butoxide (67.2g, 0.6mol) in 250g THF, slowly drop into the three-necked bottle, control the internal temperature <5°C, and complete the dropwise addition in about 0.5h. Insulate at -5°C to 5°C for 2 hours. Add 400g deionized water, stir for 10min, separate liquid, wash the organic phase with 400mL X2 water to neutral, remove toluene, add 800g petroleum ether, stir for 0.5h, filter with suction, rinse with 200g petroleum ether, collect the filtrate, remove petroleum ether 82.2 g of 8-(2-methoxy-vinyl)quinoline was obtained, the yield was 88.8%, MS (m / s): 185.1.
Embodiment 2
[0054] Synthesis of Quinoline-8-Etaldehyde (Compound 2)
[0055] In a 1L three-neck flask, add 8-(2-methoxy-vinyl)quinoline (74g, 0.4mol), 170gTHF, N 2 Protection, stirring for 20 minutes, adding 190g of concentrated hydrochloric acid to 150g of water to dilute, then slowly dripping into a three-necked bottle, controlling the internal temperature 2 Cl 2 , stirred for 5min, separated, washed the organic phase with 150mLX2 water, 40g anhydrous Na 2 SO 4 After drying, filtering, and removing the solvent, 69 g of quinoline-8-acetaldehyde was obtained, with a yield of 101%. The obtained product was directly used in the next reaction without further purification.
Embodiment 3
[0057] Synthesis of 7,8,9,10-tetrahydronaphtho[1,2-h]quinoline (compound 3)
[0058] In a 3L three-necked flask, add quinoline-8-acetaldehyde (69g) prepared in the previous step, cyclohexenyl trimethylsilyl ether (81.5g, 0.48mol), dichloromethane (2000g), and cool down to the internal temperature 2 SO 4 Dry, filter, and collect the filtrate. The filtrate quickly passes through a 100g basic alumina column, collects the column liquid, and removes the solvent to obtain a crude product. Using ethyl acetate:petroleum ether=1:1 as the eluent, silica gel column chromatography, 32.5 g of the target product was obtained, with a yield of 34.8%, MS (m / s): 233.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com