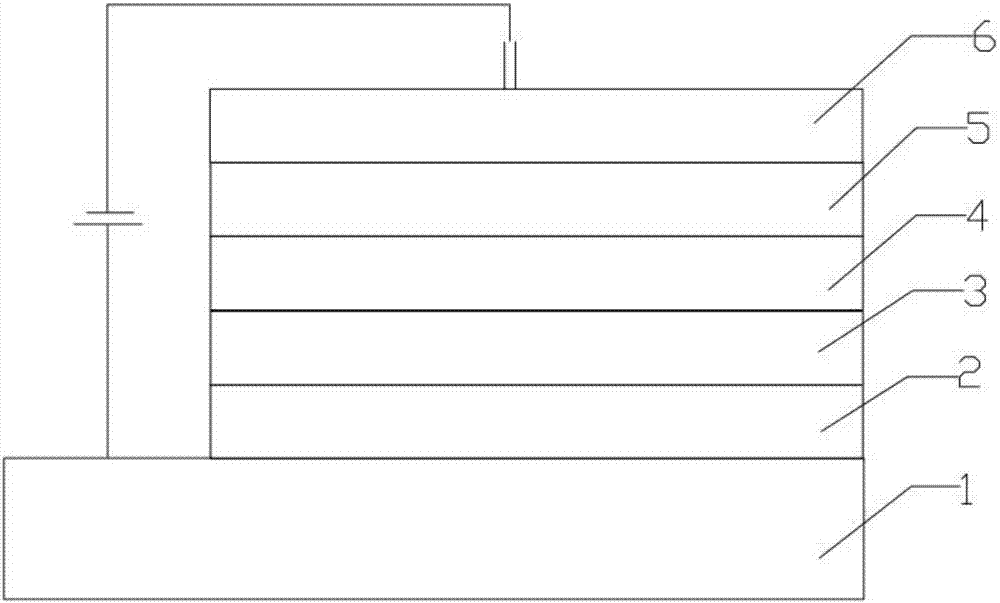
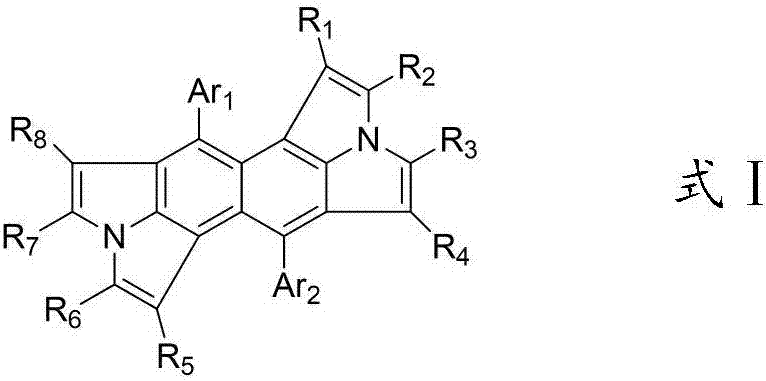
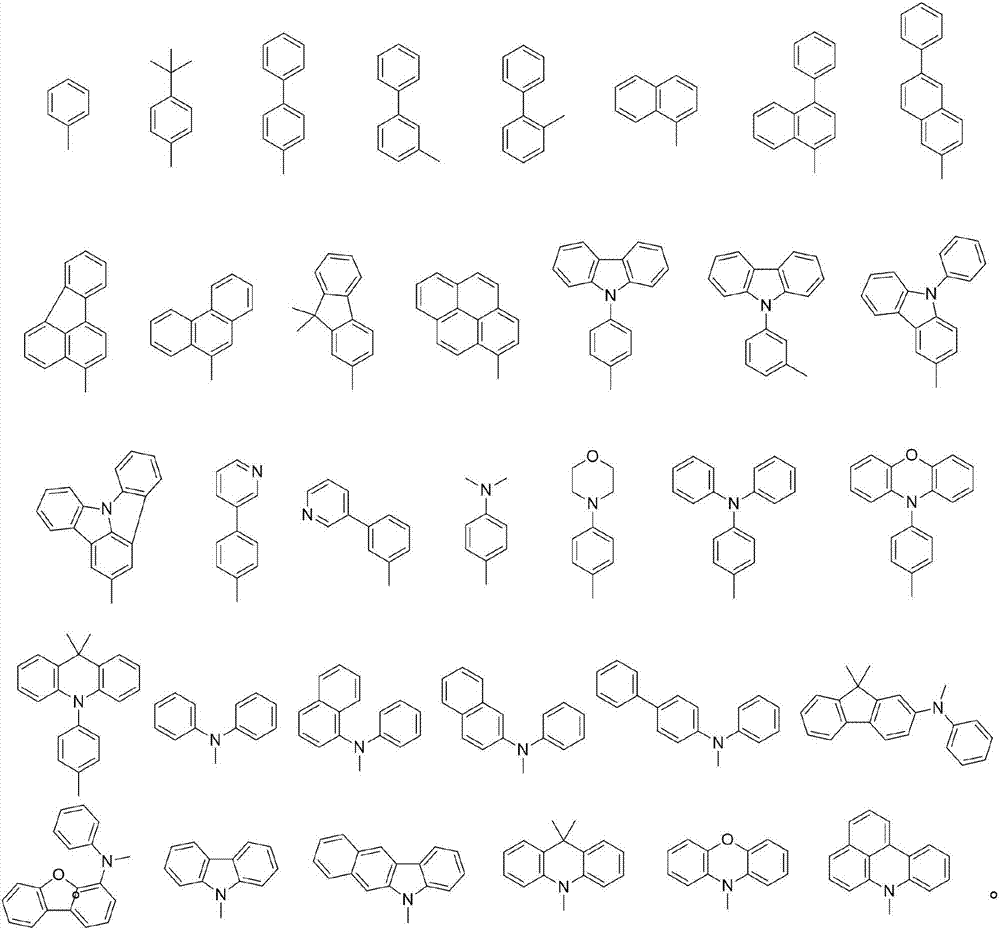
Organic electroluminescence material employing double pyrrolo-indole as core and application of organic light-emitting material
A luminescence and bispyrrole technology, applied in the field of organic electroluminescent materials, can solve the problems of low device life and efficiency, and achieve the effects of long service life, high efficiency, and good film stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 compound C01
[0033] 1) react with raw material 1 to generate intermediate 1, the reaction formula is as follows:
[0034]
[0035] The specific reaction process is: in a 2L three-necked flask, add raw material 1 (127g, 0.5mol), DMF (1050g), at 25°C, add N-bromosuccinimide (195g, 1.1mol) in batches, 1.5 After the addition of h, keep the reaction at 25°C for 4h, raise the temperature to 40°C, hold the reaction for 1h, cool down to 25°C, slowly pour the reaction solution into 3L deionized water, stir for 1h, filter with suction, rinse with 1.5L deionized water, The filter cake was collected, purified by a silica gel column, and eluted with ethyl acetate:petroleum ether=1:3 (V / V), and further recrystallized with isopropanol to obtain 117 g of the fine product of Intermediate 1 with a yield of 56%. High resolution mass spectrometry, positive ion mode, molecular formula C 18 h 8 Br 2 N 2 , the theoretical value is 411.9034, and the te...
Embodiment 2
[0039] The preparation of embodiment 2 compound C03
[0040] Using intermediate 1 and 4-biphenylboronic acid as raw materials, according to the method described in Example 1 and the following reaction formula, compound C03 was synthesized with a yield of 67%.
[0041]
[0042] High resolution mass spectrometry, positive ion mode, molecular formula C 42 h 26 N 2 , the theoretical value is 558.2096, and the test value is 558.2088. Elemental analysis (C 42 h 26 N 2 ), theoretical value C: 90.29, H: 4.69, N: 5.01, measured value C: 90.31, H: 4.66, N: 5.03.
Embodiment 3
[0043] The preparation of embodiment 3 compound C04
[0044] Using intermediate 1 and 1-naphthylboronic acid as raw materials, according to the method described in Example 1 and the following reaction formula, compound C04 was synthesized with a yield of 60%.
[0045]
[0046] High resolution mass spectrometry, positive ion mode, molecular formula C 38 h 22 N 2 , theoretical value 506.1783, test value 5061787. Elemental analysis (C 38 h 22 N 2 ), theoretical value C: 90.09, H: 4.38, N: 5.53, measured value C: 90.11, H: 4.36, N: 5.53.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com