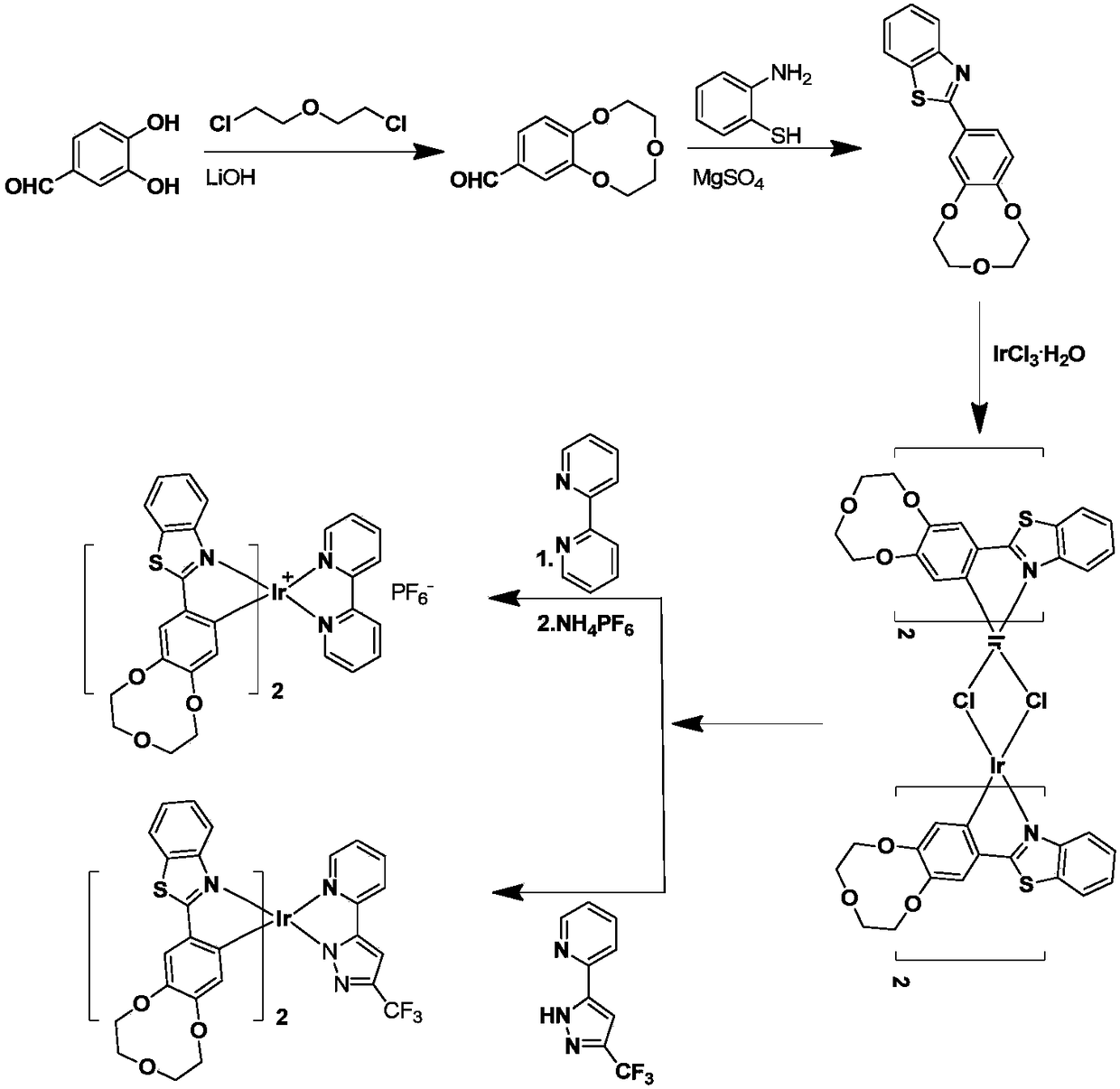
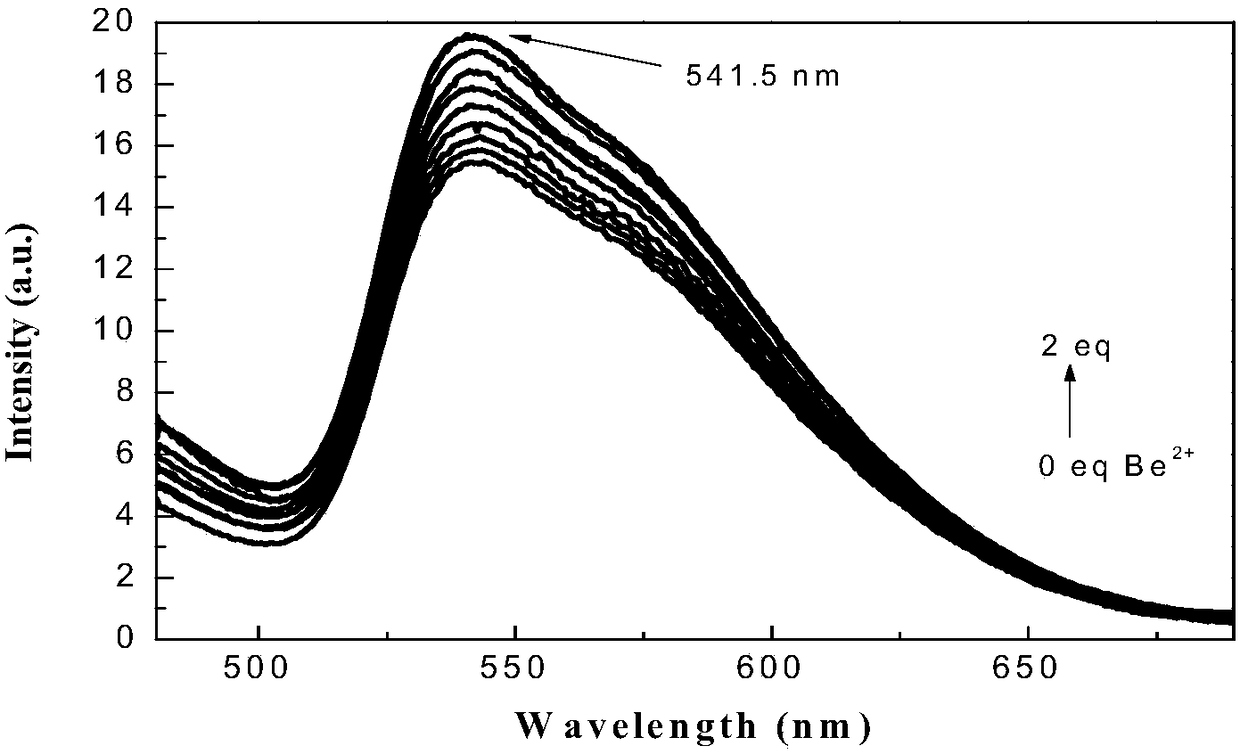
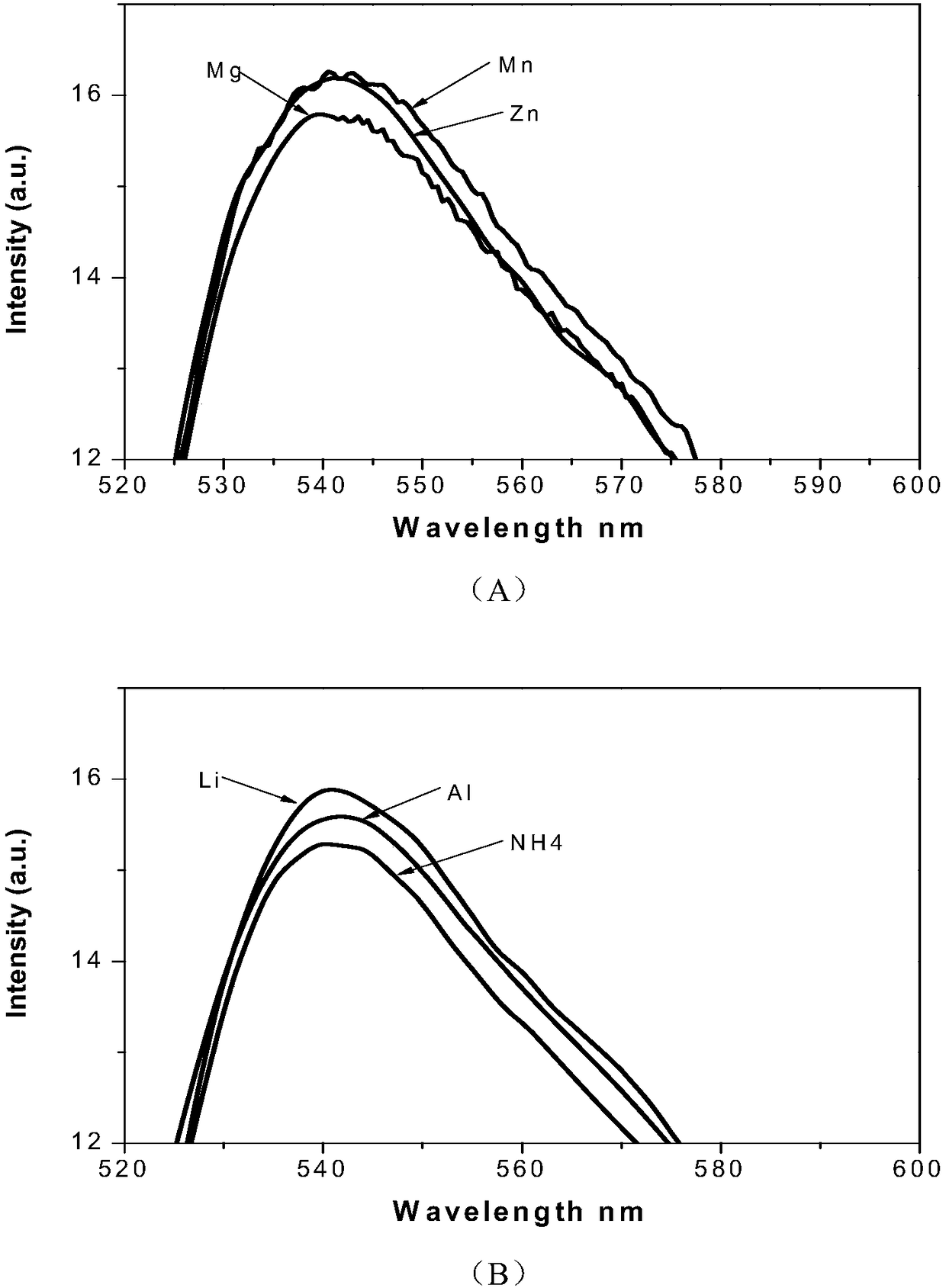
Iridium complex-containing phosphor material as well as preparation and application in beryllium ion detection
A phosphorescent material, iridium complex technology, applied in luminescent materials, material analysis by optical means, material analysis and other directions, can solve problems such as inability to detect beryllium ions, achieve high anti-interference performance, simple synthesis route, high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] A preparation method of phosphorescent material containing iridium complex, the preparation method is as follows:
[0061] (1) The synthetic route of 9-crown-3 derivatives: 3,4-dihydroxybenzaldehyde and lithium hydroxide are dissolved in water, then 1,4-dichloroethyl ether solution is added dropwise thereto, under nitrogen condition, The reaction temperature is 100-120°C (100°C, 102°C, 104°C, 106°C, 108°C, 110°C, 112°C, 114°C, 116°C, 118°C, 120°C and other values can be selected for specific applications), Reaction 48-86h (48h, 50h, 52h, 54h, 56h, 58h, 60h, 62h, 64h, 66h, 68h, 70h, 72h, 74h, 76h, 78h, 80h, 82h, 84h, 86h, etc. can be selected for specific applications Numerical value) obtains the derivative of 9-crown-3;
[0062]
[0063]The amount of the reactant is in molar parts, 1 part of 3,4-dihydroxybenzaldehyde, 1-2 parts of 1,4-dichloroethyl ether (1 part, 1.1 part, 1.2 part, 1.3 part can be selected in specific application , 1.4 parts, 1.5 parts, 1.6 part...
Embodiment 1
[0079] Synthesis of 9-Crown-3 Derivatives (9C3)
[0080] Dissolve 2.4g of 3,4-dihydroxybenzaldehyde (17.4mmol) in 250mL of water, then add dropwise 1.43g (59.7mmol) of lithium hydroxide, 2ml (17.4mmol) of 1,4-dichloroethyl ether, dropwise Reflux for 86 hours after the addition, add a dilute solution of hydrochloric acid to adjust the pH to about 2, then repeatedly extract with dichloromethane and water several times, and wash the organic phase with 4% NaOH aqueous solution after washing; dry after washing, spin Dry, and finally use petroleum ether ethyl acetate system silica gel column chromatography to obtain a yellow oily product with a yield of 17.9%.
[0081] 1 H NMR (400MHz, CDCl 3 )δ9.87(s,1H),7.57(d,J=2.0Hz,1H),7.54(dd,J=8.3,2.1Hz,1H),7.09(d,J=8.2Hz,1H),4.70– 4.64(m,2H),4.35–4.28(m,2H),4.00–3.94(m,2H),3.94–3.89(m,2H).
Embodiment 2
[0083] Synthesis of 2-Benzothiazole-9-crown-3
[0084] 0.5g (2.4mmol) the product that embodiment 1 makes is dissolved in 10mL N, N dimethylformamide, drips into 0.6mL (5.6mmol) 2-aminothiophenol solution again, anhydrous magnesium sulfate 0.552g ( 4.6mmol) was reacted for 24h under the protection of nitrogen. After the reaction was completed, it was cooled to room temperature, back-extracted with water and ethyl acetate; Yield 40.8%.
[0085] 1 H NMR (400MHz, CDCl 3 )δ8.03(d, J=8.7Hz, 1H), 7.87(d, J=9.2Hz, 1H), 7.77(s, 1H), 7.68(d, J=11.0Hz, 1H), 7.41(d, J=46.9Hz, 2H), 7.06(d, J=8.4Hz, 1H), 4.52(d, J=6.7Hz, 2H), 4.38(s, 2H), 4.11(d, J=7.1Hz, 1H) ,3.99–3.86(m,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com