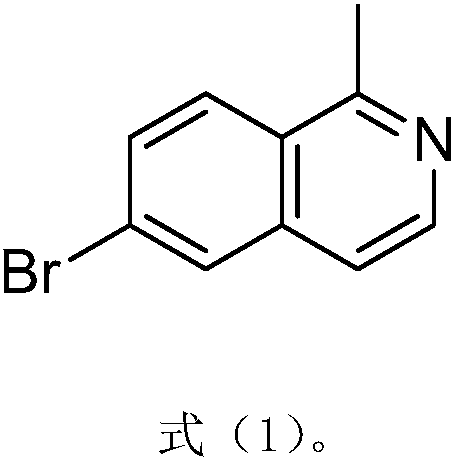
Method for synthesizing pharmaceutical intermediate nitrogen heterocyclic bromo-compound
A technology for intermediates and compounds, applied in the field of pharmaceutical intermediates, can solve problems such as inability to popularize, and achieve the effects of saving synthesis steps, high yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
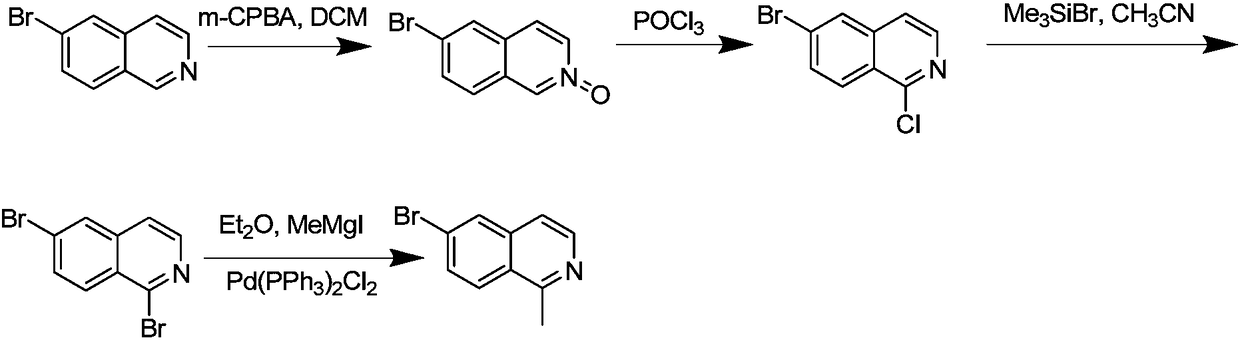
[0021] S1: Raw material preparation 200 grams of 6-bromoisoquinoline, 1.5 L of dichloromethane and 300 grams of m-chloroperoxybenzoic acid with an effective content of 80%, select a 4 L four-necked bottle with a water separator, add the aforementioned two After methyl chloride, keep stirring at 25-30°C, slowly add 6-bromoisoquinoline, and then add m-chloroperoxybenzoic acid with an effective content of 80%, keep stirring at 25-30°C overnight , after TLC showed that the reaction was complete during the day, the system was filtered with suction, and the filter cake was slowly dried and collected carefully to obtain a product mixture of step S1 containing 6-bromoisoquinoline nitrogen oxide.
[0022] S2: Take 150 g of the product mixture of the S1 step, select a 4L four-necked bottle with a water separator, add 400 ml of phosphorus oxychloride and stir, slowly add the product mixture of the S1 step into 400 ml of phosphorus oxychloride in batches, three After the phosphorus oxychl...
Embodiment 2
[0028] S1: Raw material preparation 250 grams of 6-bromoisoquinoline, 2 L of dichloromethane and 350 grams of m-chloroperoxybenzoic acid with an effective content of 85%, select a 4 L four-necked bottle with a water separator, add the aforementioned dichloro After methane, keep stirring at 25-30°C, slowly add 6-bromoisoquinoline, then add m-chloroperoxybenzoic acid with an effective content of 85%, keep stirring at 25-30°C overnight, After TLC showed that the reaction was complete during the day, the system was filtered with suction, and the filter cake was slowly dried and collected carefully to obtain the product mixture of step S1, 6-bromoisoquinoline nitrogen oxide.
[0029] S2: Take 200 g of the product mixture 6-bromoisoquinoline nitrogen oxide of step S1, select a 4L four-necked bottle with a water separator, add 500ml of phosphorus oxychloride and stir, and mix the mixture 6-bromoisoquinoline Nitrogen oxides were slowly added to 500ml of phosphorus oxychloride in batch...
Embodiment 3
[0035] S1: Raw material preparation 250 grams of 6-bromoisoquinoline, 2 L of dichloromethane and 350 grams of m-chloroperoxybenzoic acid with an effective content of 85%, select a 4 L four-necked bottle with a water separator, add the aforementioned dichloro After methane, keep stirring at 25-30°C, slowly add 6-bromoisoquinoline, then add m-chloroperoxybenzoic acid with an effective content of 85%, keep stirring at 25-30°C overnight, After TLC showed that the reaction was complete during the day, the system was filtered with suction, and the filter cake was slowly dried and collected carefully to obtain the product mixture of step S1, 6-bromoisoquinoline nitrogen oxide.
[0036] S2: Take 200 g of the product mixture 6-bromoisoquinoline nitrogen oxide of step S1, select a 4L four-necked bottle with a water separator, add 500ml of phosphorus oxychloride and stir, and mix the mixture 6-bromoisoquinoline Nitrogen oxides were slowly added to 500ml of phosphorus oxychloride in batch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com