Method for synthesizing butenafine
A butenafine and synthetic method technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low reaction yield and many side reactions, and achieve the effects of simple steps, convenient operation and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
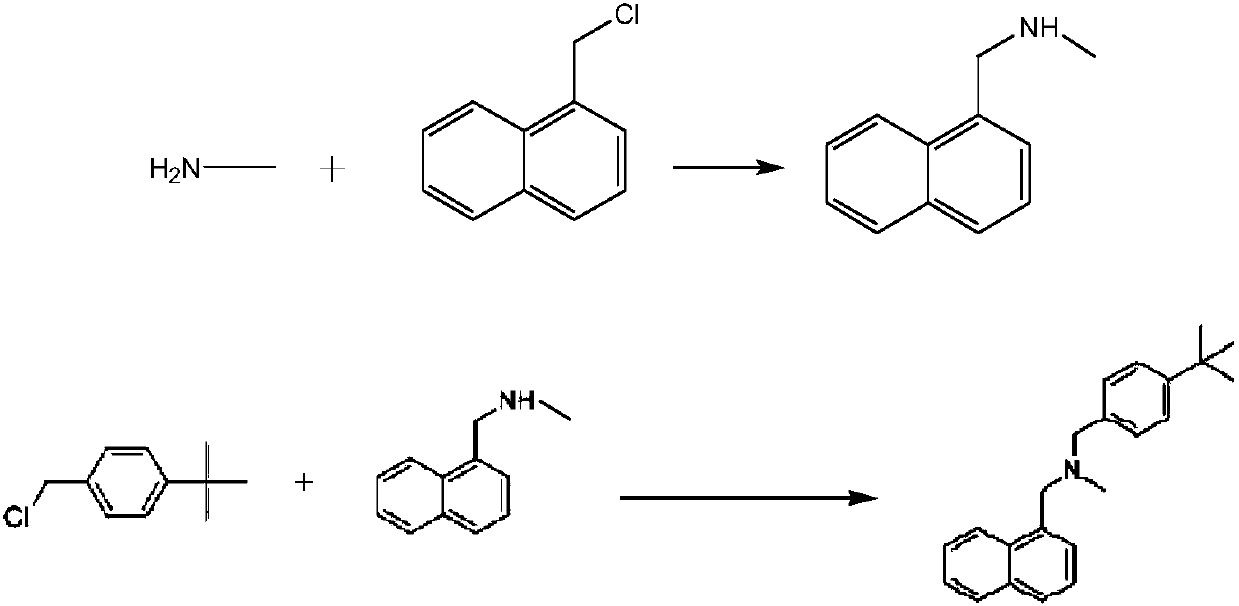
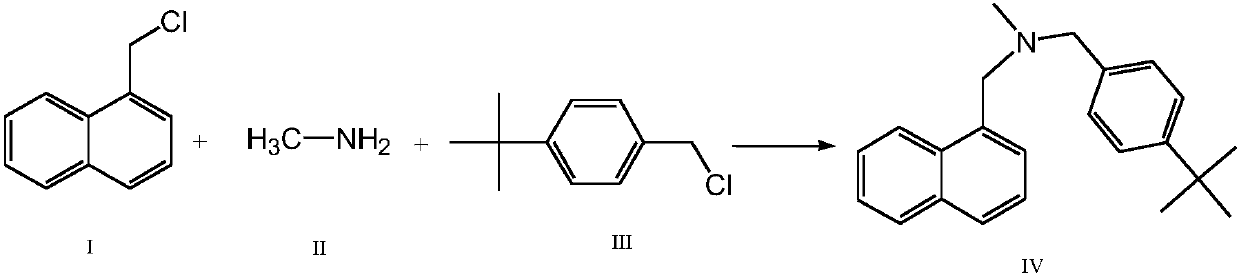
[0025] Embodiment 1: add purified water 1000ml in 2000ml reaction bottle, slowly add 36g monomethylamine in water, then add salt of wormwood 312g in reaction bottle, add 1-chloromethyl naphthalene 100g respectively in two constant pressure funnels, 109 g of p-tert-butyl benzyl chloride, temperature control 10-20 ° C, slowly drop 1-chloromethylnaphthalene and p-tert-butyl benzyl chloride at the same time, control the rate of addition, so that 1-chloromethyl naphthalene and p-tert-butyl chloride The benzyl was added dropwise at the same time, and then the temperature was controlled at 10-20°C and the reaction was kept for 4 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C and concentrate the organic layer to dryness under reduced pressure, add 480ml of isopropanol to the residue, heat to 60°C, stir for 30 minutes, cool down to below 10°C, Crystallize for 30 minutes, filter with suction to ...
Embodiment 2
[0026] Example 2: Add 1000ml of purified water to a 2000ml reaction flask, slowly add 40g of monomethylamine to the water, then add 353.8g of potassium carbonate to the reaction flask, and add 110.5 g of 1-chloromethylnaphthalene to two constant pressure funnels g, p-tert-butyl benzyl chloride 116.9g, temperature control 10-20 ℃, slowly drop 1-chloromethylnaphthalene and p-tert-butyl benzyl chloride at the same time, control the rate of addition, make 1-chloromethyl naphthalene and p-tert The butyl benzyl chloride was all added dropwise at the same time, and then the temperature was controlled at 10-20°C and the reaction was kept for 4 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C and concentrate the organic layer to dryness under reduced pressure, add 480ml of isopropanol to the residue, heat to 60°C, stir for 30 minutes, cool down to below 10°C, Crystallize for 30 minutes, filter wi...
Embodiment 3
[0027] Embodiment 3: Add purified water 1000ml in 2000ml reaction bottle, slowly add 38g monomethylamine in water, then add potassium carbonate 328.5g in reaction bottle, add 1-chloromethyl naphthalene 103g respectively in two constant pressure funnels , p-tert-butyl benzyl chloride 111g, temperature control 10-20 ° C, slowly drop 1-chloromethylnaphthalene and p-tert-butyl benzyl chloride at the same time, control the rate of addition, so that 1-chloromethyl naphthalene and p-tert-butyl The benzyl chloride was all added dropwise at the same time, and then the temperature was controlled at 10-20°C and the reaction was kept for 4 hours. Add 500ml of chloroform to the reaction solution to extract the reaction solution, control the temperature of the water bath at 60°C and concentrate the organic layer to dryness under reduced pressure, add 480ml of isopropanol to the residue, heat to 60°C, stir for 30 minutes, cool down to below 10°C, Crystallize for 30 minutes, filter with sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com