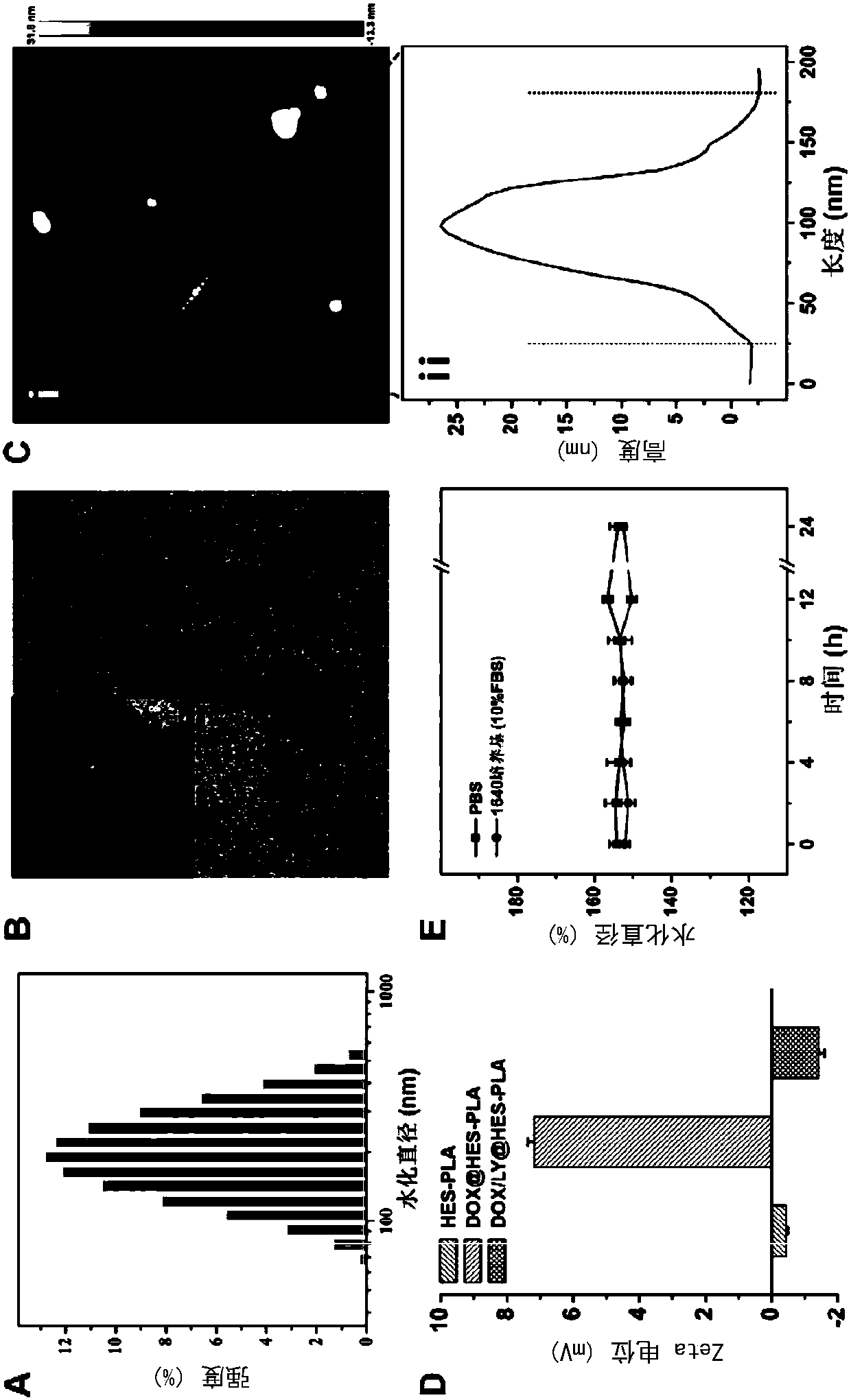
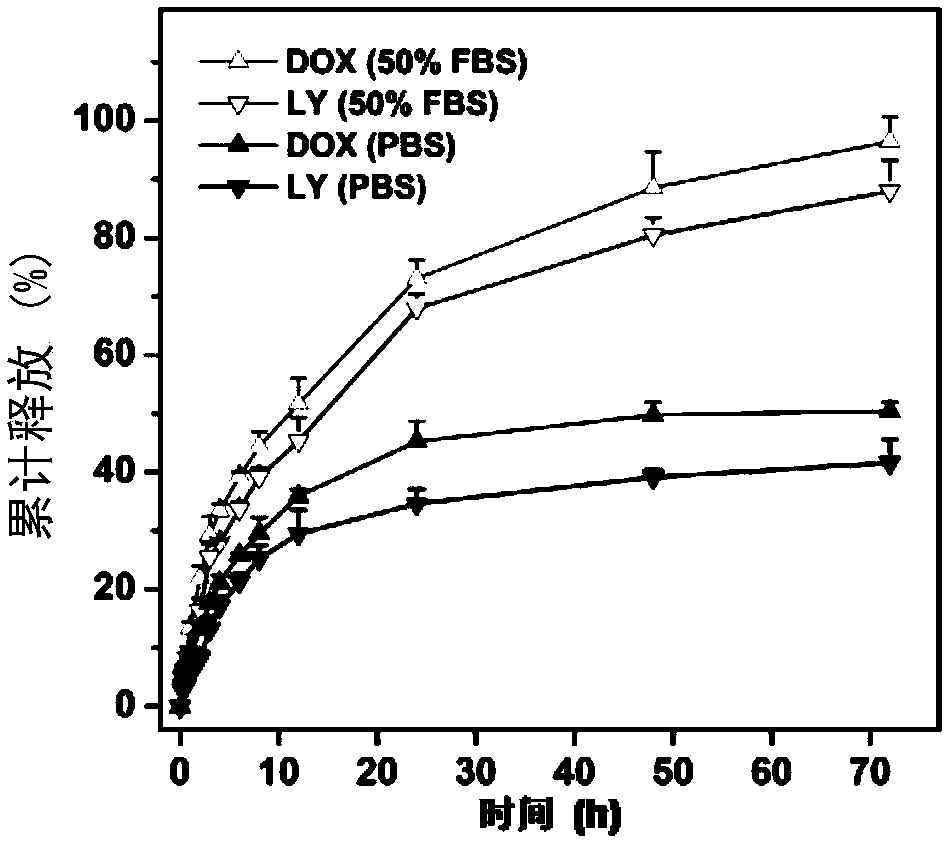
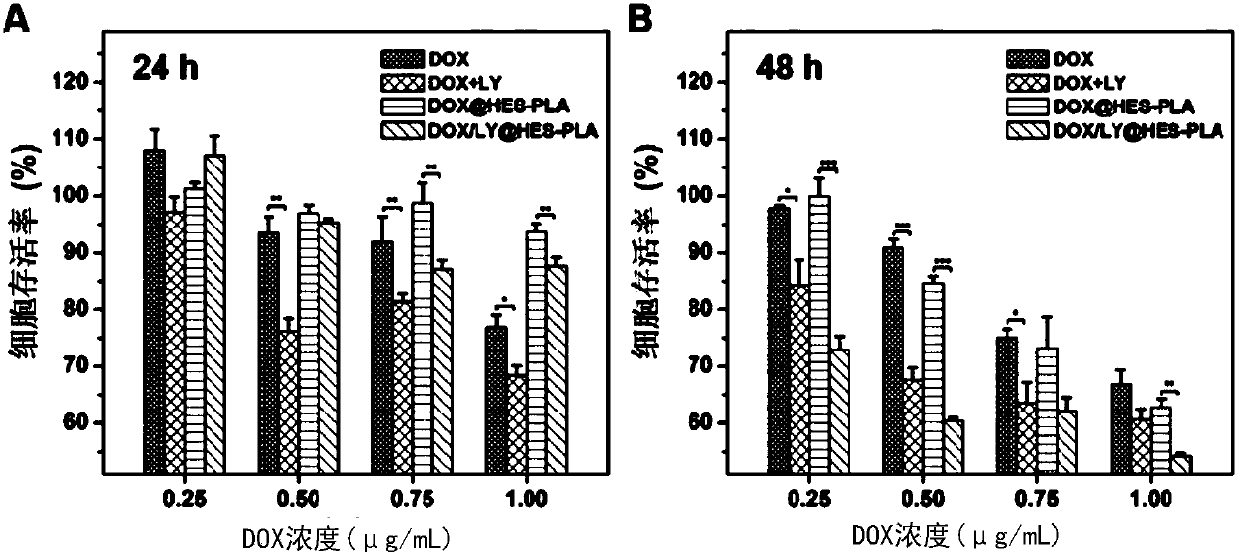
Nanometer drug carrying system and preparation method thereof as well as pharmaceutical composition and application of co-encapsulated nanometer drug carrying system to cancer treatment
A nano-drug-carrying and nano-carrier technology, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as aggravation of EMT, invasion and metastasis, and achieve the effect of preventing invasion and metastasis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The preparation method of the HES-PLA graft copolymer can be referred to, for example, the method disclosed in the patent application CN103467753A, which is incorporated herein by reference in its entirety.
[0088] According to a preferred embodiment, the preparation method of the nanometer drug-carrying system of the present invention using HES-PLA graft polymer as carrier comprises:
[0089] Specifically include the following steps:
[0090] (1) Dissolving PLA and activating its terminal carboxyl group: Add catalyst N-N'-dicyclohexylcarbodiimide and 4-dimethylaminopyridine to the carboxyl-terminated PLA, with anhydrous dimethyl sulfoxide as solvent, 50 React at ~70°C for 25 to 45 minutes to dissolve it completely to obtain PLA with activated carboxyl groups; the molecular weight of the PLA is 10 to 30 kDa, preferably 10 kDa, and the PLA, N-N'-dicyclohexylcarbodi The molar ratio of imine and 4-dimethylaminopyridine is 1:4:2;
[0091] (2) Dissolving HES: Under nitrog...
Embodiment 1
[0155] The preparation of embodiment 1HES-PLA copolymer
[0156] Weigh 0.5g of HES (Hua Keda Life Science and Technology Co., Ltd., hydroxyethylation rate: 0.5) and put it in a drying oven at 105°C for 2 hours, take it out, and dissolve it in 20mL DMSO (60°C). At the same time, PLA (Mw 5kDa, Jinan Daigang Bioengineering Co., Ltd.), dicyclohexylcarbodiimide and dimethylaminopyridine were dissolved in 10 mL of DMSO at a molar ratio of 1:4:2. After the two solutions were mixed, they were placed in a round-bottomed flask under nitrogen protection, and the temperature was maintained at 60° C. in an oil bath, and the reaction was stirred for 24 hours. After the reaction, the product was collected, placed in a dialysis bag with a molecular weight cut-off of 3500, and dialyzed with ultrapure water for 3 days. After lyophilization, the dialyzed product was purified by adding dichloromethane through a Soxhlet extractor (70° C., 24 h) to remove unreacted PLA. The product obtained after...
Embodiment 2
[0157] Example 2 Preparation of nanoparticles (DOX / LY@nanoparticles) co-encapsulating DOX and LY2157299 (hereinafter referred to as LY)
[0158] Weigh 6mg of DOX hydrochloride (Beijing Huafeng Lianbo Co., Ltd.), and suspend it in 1mL of chloroform. According to the molar ratio DOX:triethylamine=1:3, triethylamine was added to the suspension. After the suspension was sonicated for 1 h, centrifuged at 3000 rpm for 10 min, the supernatant was taken, which was the DOX chloroform solution depleted of hydrochloric acid. Then 12 mg LY was weighed, added to the DOX chloroform solution, and vortexed until there were no obvious particles in the solution.
[0159]Weigh 30 mg of the HES-PLA copolymer prepared in Example 1 and dissolve it in 20 mL of ultrapure water. In an ice bath, the above 1 mL DOX / LY chloroform solution was added dropwise to the HES-PLA aqueous solution. During the dropwise addition, the solution was emulsified by a cell ultrasonic breaker (Ningbo Xinzhi Biotechnolo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com