Preparation method of tildipirosin
A new and independent technology of Taidiluo, applied in the field of medicine, can solve the problems of many by-products and unsuitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention provides a new preparation method of tedrol, comprising the following steps:
[0027] (1) Mixing tylosin, piperidine, a reducing agent and an organic solvent, and performing the first amination reaction to obtain compound I;
[0028] (2) Mix the compound I, acid and water, and carry out a neutralization reaction to obtain a salt solution;
[0029] (3) After stepwise acid hydrolysis of the salt solution, alkalization treatment is carried out to obtain compound II;
[0030] (4) Mixing the compound II, oxidant, and organic solvent, and performing an oxidation reaction to obtain compound III;
[0031] (5) mixing the compound III, piperidine, a reducing agent and an organic solvent, and performing a second amination reaction to obtain tedirosin;
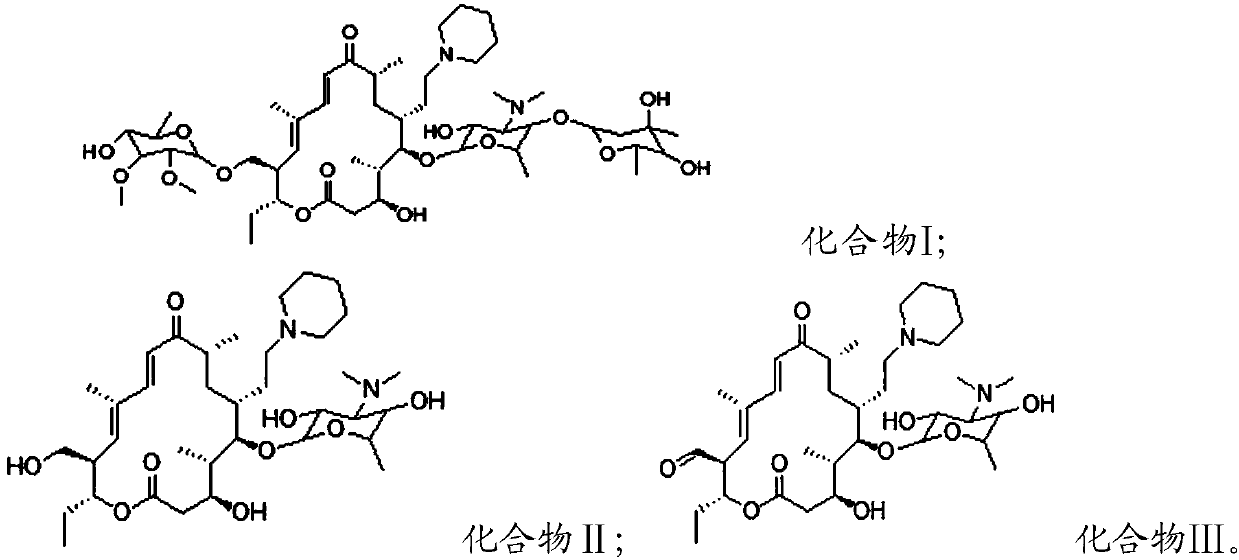
[0032] The structures of Compound I, Compound II and Compound III are as follows:
[0033] Compound I;
[0034] Compound II; Compound III.
[0035] In the present invention, compound I is obtained b...
Embodiment 1
[0095] (1) Mix 1000g (1.1mol) of tylosin, 468g of piperidine and 5L of butyl acetate, heat up to 50°C, add 25g of formic acid dropwise, and carry out the amination reaction; 3L of deionized water was added to quench the reaction, and the phases were separated to obtain an organic phase containing compound I;
[0096] (2) Mix the organic phase containing compound I with 5L of deionized water, adjust the pH value of the system to 2 using dilute sulfuric acid with a mass concentration of 30%, and separate the phases to obtain a salt solution;
[0097] (3) in described salt solution, adding mass concentration is 50% dilute sulfuric acid 250ml, and the mol ratio of described sulfuric acid and tylosin is 1.2:1, stirs at room temperature and carries out preliminary hydrolysis, and the time of described preliminary hydrolysis is 1h; after the completion of the preliminary hydrolysis, add 160ml of concentrated sulfuric acid with a mass concentration of 80%, the molar ratio of the sulfu...
Embodiment 2
[0103] (1) Mix 1000g (1.1mol) tylosin, 468g piperidine and 5L isopropyl acetate, heat up to 53°C, keep the system temperature constant, add 60g sodium cyanoborohydride in batches, and carry out amination reaction ; After reacting for 1 hour, cool down to room temperature, add 3L deionized water to the system to quench the reaction, and separate phases to obtain an organic phase containing compound I;
[0104] (2) Mix the organic phase containing compound I with 5L of deionized water, adjust the pH value of the system to 3 by using dilute sulfuric acid with a mass concentration of 30%, and separate the phases to obtain a salt solution;
[0105] (3) in described salt solution, adding mass concentration is 50% dilute sulfuric acid 250ml, adds 2g hydrobromic acid simultaneously, and the mol ratio of described sulfuric acid and tylosin is 1.2:1, stirs at room temperature and carries out preliminary hydrolysis, The time for the preliminary hydrolysis is 1.5h; after the completion of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com