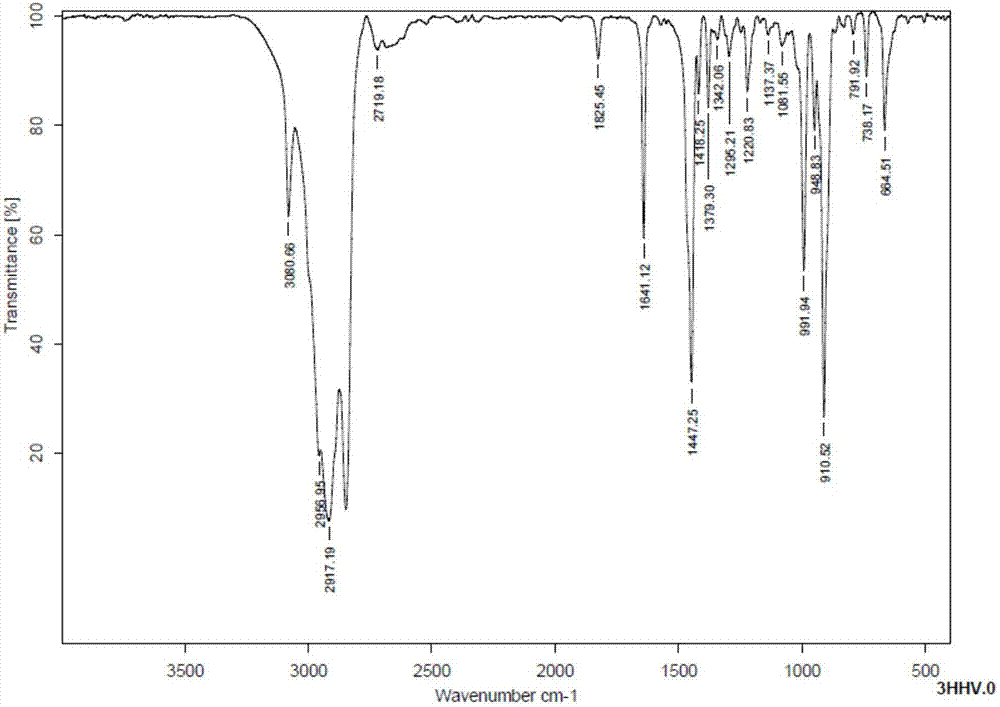
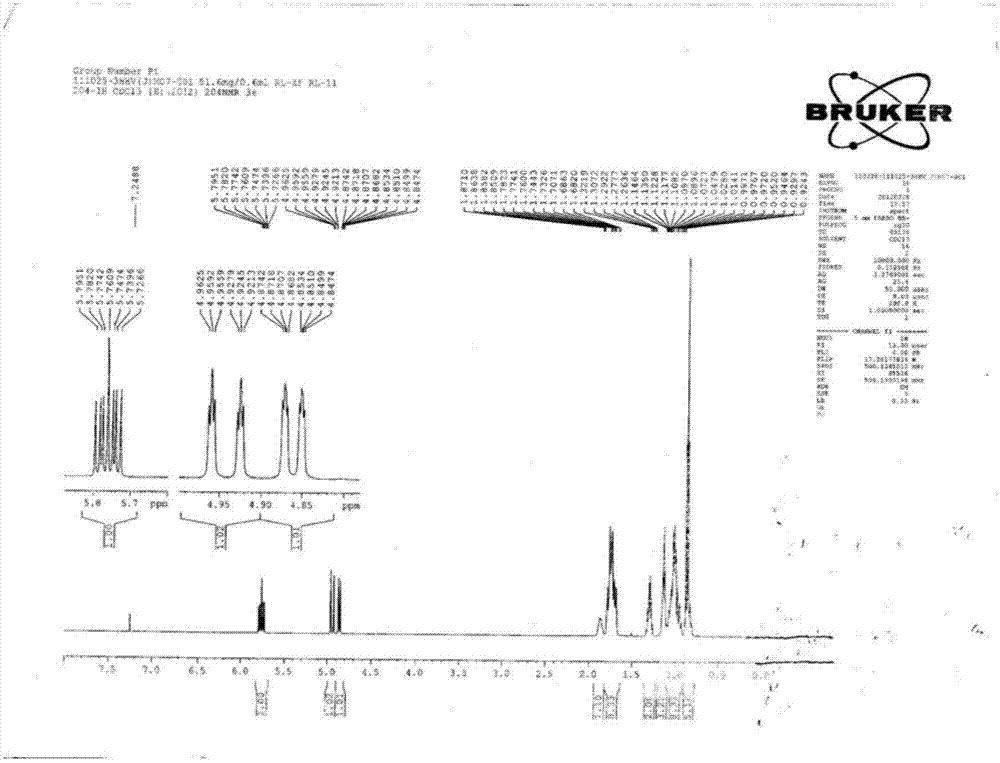
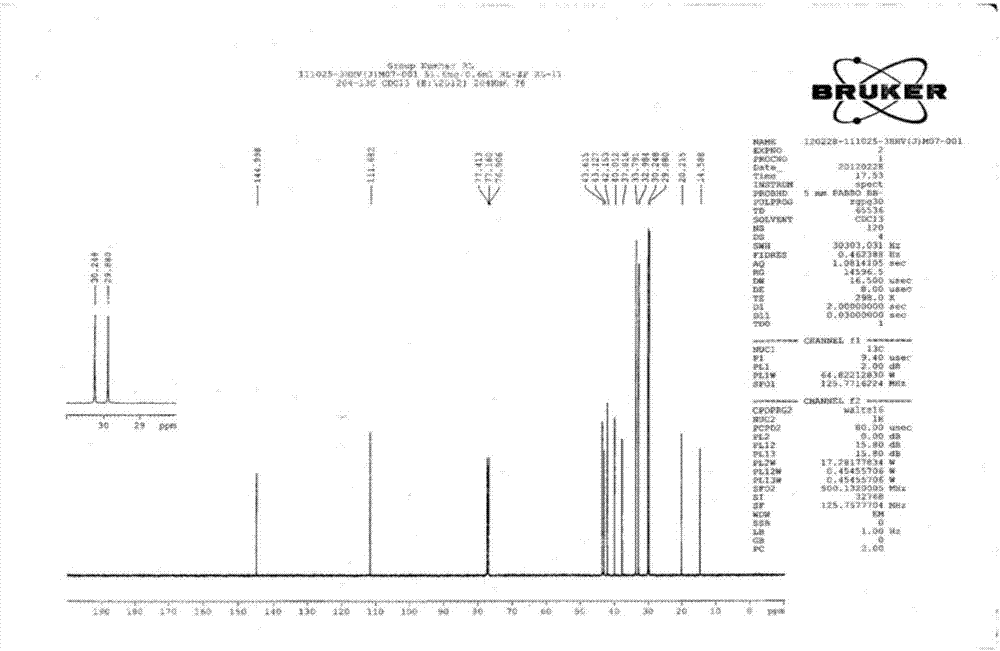
Method for synthesizing trans-4-(trans-4'-alkylcyclohexyl)cyclohexylethylene liquid crystal monomer
A technology of cyclohexylethylene and a synthesis method, which is applied in the synthesis field of trans-4-cyclohexylethylene liquid crystal monomer, can solve the problems of high cost, low atom utilization rate, large consumption of raw materials, etc., and achieves low cost and low avoidance. Atomic utilization, effect of avoiding environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of compound (A)
[0053] Under the protection of nitrogen, add 2520ml of methanol, 420.0g of trans-4-(trans-4'-n-propylcyclohexyl)cyclohexyl ethyl ketone, and 72.0g of p-toluenesulfonic acid to a dry 10L three-necked flask, and cool the system down to 20°C At ~25°C, add 357.0g of N-bromosuccinimide in batches, control the temperature at 30°C to 40°C, react for 3 hours after the addition, and stop the reaction. After concentration and recovery of methanol under reduced pressure, 2100ml of water was added, and solids were precipitated after stirring. The solids were filtered and dried to obtain 526.3g of compound A, GC>96%, and the yield was 95%.
[0054] (2) Preparation of compound (B)
[0055] Add 2770ml of methanol and 554.0g of compound A to a 10L three-necked flask successively, add 32.0g of sodium borohydride in batches, start heating to the reaction temperature of 64-97°C, reflux for 5 hours, distill out the methanol system, and pour the concentrat...
Embodiment 2
[0063] (1) Preparation of compound (A)
[0064] Under the protection of nitrogen, add 2520ml of methanol, 420.0g of trans-4-(trans-4'-n-propylcyclohexyl)cyclohexyl ethyl ketone, and 72.0g of p-toluenesulfonic acid to a dry 10L three-necked flask, and cool the system down to 20°C ~25°C, add 258.0g dibromohydantoin in batches, control the temperature at 20°C~30°C, react for 3 hours after the addition, stop the reaction, concentrate under reduced pressure to recover methanol, add 2100ml water to the concentrate and stir, and wash the precipitated solid with water until Neutral, 520.0g compound A was obtained by drying, GC>96%, yield 93.8%.
[0065] (2) Preparation of compound (B)
[0066] Add 4160ml of ethanol and 520.0g of compound (A) to a 10L three-necked flask in sequence, add 28.5g of sodium borohydride in batches, heat to a reaction temperature of 64-97°C and reflux for 4 hours, distill off the system ethanol, pour the concentrated solution into 300ml 15 % dilute hydrochl...
Embodiment 3
[0074] (1) Preparation of compound (A)
[0075] Under the protection of nitrogen, add 4000ml of methanol, 500.0g of trans-4-(trans-4'-n-propylcyclohexyl)cyclohexyl ethyl ketone, and 50.0g of p-toluenesulfonic acid to a dry 10L three-necked flask, and cool the system down to 10°C ~20°C, add 343.0g dibromohydantoin in batches, each batch interval is 10min, control temperature 10°C~20°C, react for 1h after feeding, stop reaction, concentrate under reduced pressure to recover methanol, add and stir to the concentrate, the precipitated The solid was washed with water until neutral, and dried to obtain 630.0 g of compound A, GC>96%, and the yield was 95.7%.
[0076] (2) Preparation of compound (B)
[0077] Add 3120ml of ethanol and 520.0g of compound (A) to a 10L three-necked flask in turn, add 34.2g of sodium borohydride in ten batches (10 minutes between batches) and start heating to the reaction temperature of 64-97°C, reflux for 4 hours, and distill off the system ethanol , Po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com