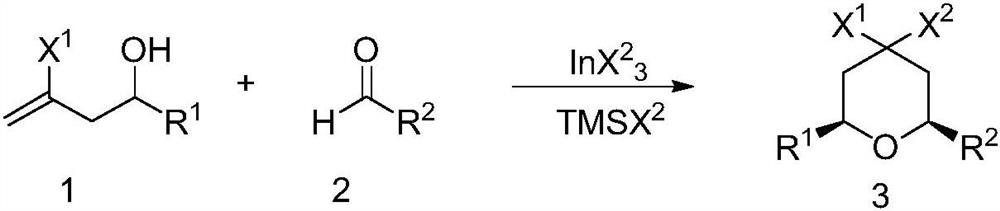
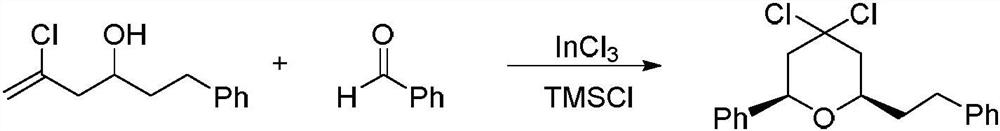A kind of preparation method of 4,4-dihalogenated tetrahydropyran
A technology of tetrahydropyran and dihalogenation, applied in 4 fields, can solve the problems of consumption of metal resources, insufficient atomic economy of indium trichloride, etc., and achieve the effects of high purity of finished products, reduction of catalytic cost, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of 2-phenylethyl-6-phenyl-4,4-dichlorotetrahydro-2H-pyran, the reaction formula is as follows:
[0024]
[0025] The method includes the following steps:
[0026] S1, 0.35 g of 5-chloro-1-phenylhex-5-en-3-ol (1.65 mmol), 0.18 g of benzaldehyde (1.65 mmol), 35 mg of indium trichloride (0.16 mmol) and 10 ml of CH 2 Cl 2 Mix in a flask and stir to form a homogeneous mixture;
[0027] S2. The mixed solution obtained in step S1 is controlled at a temperature of 0° C. by a low-temperature thermostat, and 0.2 g of trimethylchlorosilane (1.82 mmol) is added dropwise to start the reaction;
[0028] S3. Control the temperature of the reaction solution obtained in step S2 at 25-27°C, stir and react for 12 hours, add saturated sodium bicarbonate solution to quench the reaction, move the solution to a separatory funnel, and separate the organic phase and the aqueous phase from the solution ;
[0029] S4, the aqueous phase obtained in step S3 is extracte...
Embodiment 2
[0032] The preparation method of 2-phenethyl-6-phenyl-4,4-dibromotetrahydro-2H-pyran, the reaction formula is as follows:
[0033]
[0034] The method includes the following steps:
[0035] S1, 0.42 g of 5-bromo-1-phenylhex-5-en-3-ol (1.65 mmol), 0.18 g of benzaldehyde (1.65 mmol), 56 mg of indium tribromide (0.16 mmol) and 10 ml of CH 2 Cl 2 Mix in a flask and stir to form a homogeneous mixture;
[0036] S2. The mixed solution obtained in step S1 is controlled at a temperature of 0° C. by a low-temperature thermostat, and 0.28 g of bromotrimethylsilane (1.82 mmol) is added dropwise to start the reaction;
[0037] S3. Control the temperature of the reaction solution obtained in step S2 at 22-25° C., stir and react for 12 hours, add saturated sodium bicarbonate solution to quench the reaction, move the solution to a separatory funnel, and separate the organic phase and the aqueous phase from the solution ;
[0038] S4, the aqueous phase obtained in step S3 is extracted t...
Embodiment 3
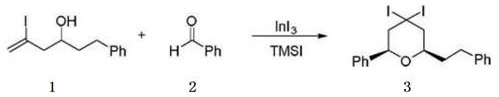
[0041]The preparation method of 2-phenethyl-6-phenyl-4,4-diiodotetrahydro-2H-pyran, the reaction formula is as follows:
[0042]
[0043] The method includes the following steps:
[0044] S1, 0.5 g of 5-iodo-1-phenylhex-5-en-3-ol (1.65 mmol), 0.18 g of benzaldehyde (1.65 mmol), 79 mg of indium triiodide (0.16 mmol) and 10 ml of CH 2 Cl 2 Mix in a flask and stir to form a homogeneous mixture;
[0045] S2. The mixed solution obtained in step S1 is controlled at a temperature of 0° C. by a low-temperature thermostat, and 0.36 g of iodotrimethylsilane (1.82 mmol) is added dropwise to start the reaction;
[0046] S3. Control the temperature of the reaction solution obtained in step S2 at 27-30°C, stir and react for 12 hours, add saturated sodium bicarbonate solution to quench the reaction, move the solution to a separatory funnel, and separate the organic phase and the aqueous phase from the solution ;
[0047] S4, the aqueous phase obtained in step S3 is extracted three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com