A Dithiothreitol Fluorescent Probe
A technology of dithiothreitol and fluorescent probes, applied in the field of analytical chemistry, can solve the problems of rare specific detection and identification of dithiothreitol molecular fluorescent probes, and achieve easy promotion, cheap raw materials, and low preparation costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of dithiothreitol fluorescent probe.
[0038] 4-Diethylaminoketoacid (1 mmol) and 1,6-dihydroxynaphthalene (1 mmol) were heated to reflux at 90°C in concentrated sulfuric acid (4 mL); then the reaction solution was cooled to 0°C, and perchloric acid ( 4 mL) to make it precipitate, filter with suction and dry to obtain a solid, dissolve the solid in methanol, fill the chromatography column with silica gel powder, use a mixed solvent of dichloromethane:methanol=10:1 as the eluent, and after elution The solution was desolvated to obtain compound 1 as a solid.
[0039] Add compound 1 (1 mmol), 2,4-dinitrobenzenesulfonyl chloride (1 mmol), triethylamine (0.2 mmol) and dichloromethane (10 mL) into a 50 mL one-necked flask and stir at 0 °C After 2 hours, the reaction was complete. After spin-drying, the obtained solid was purified by column chromatography, and the light red product obtained was the fluorescent probe for detecting dithiothreitol (DTT) acc...
Embodiment 2
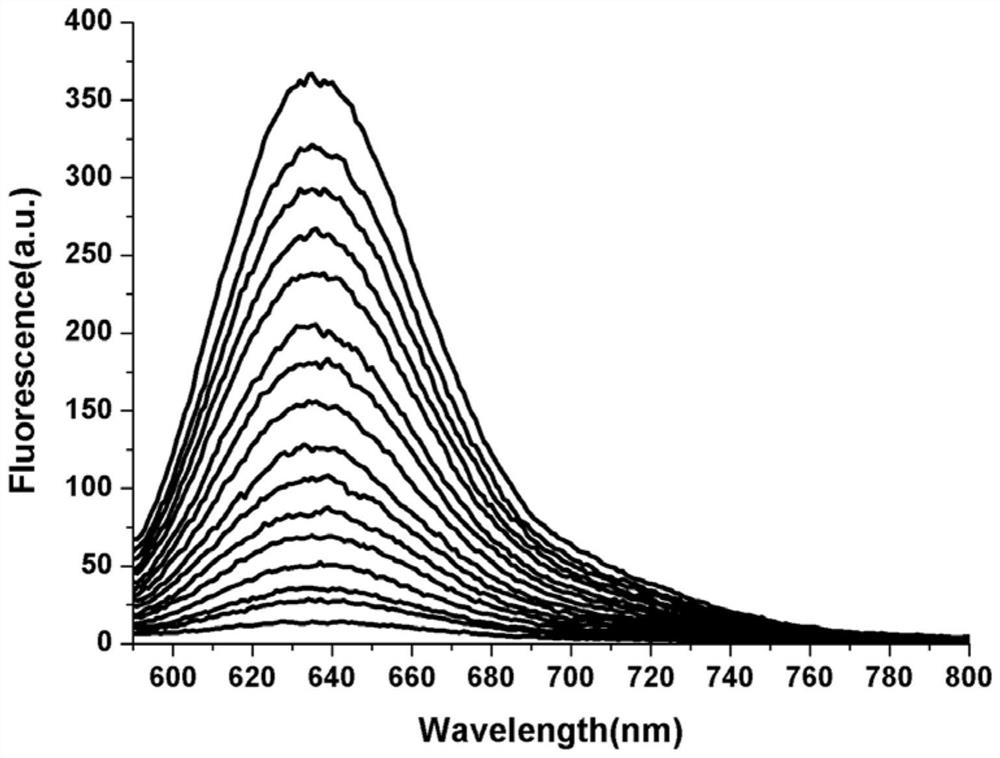
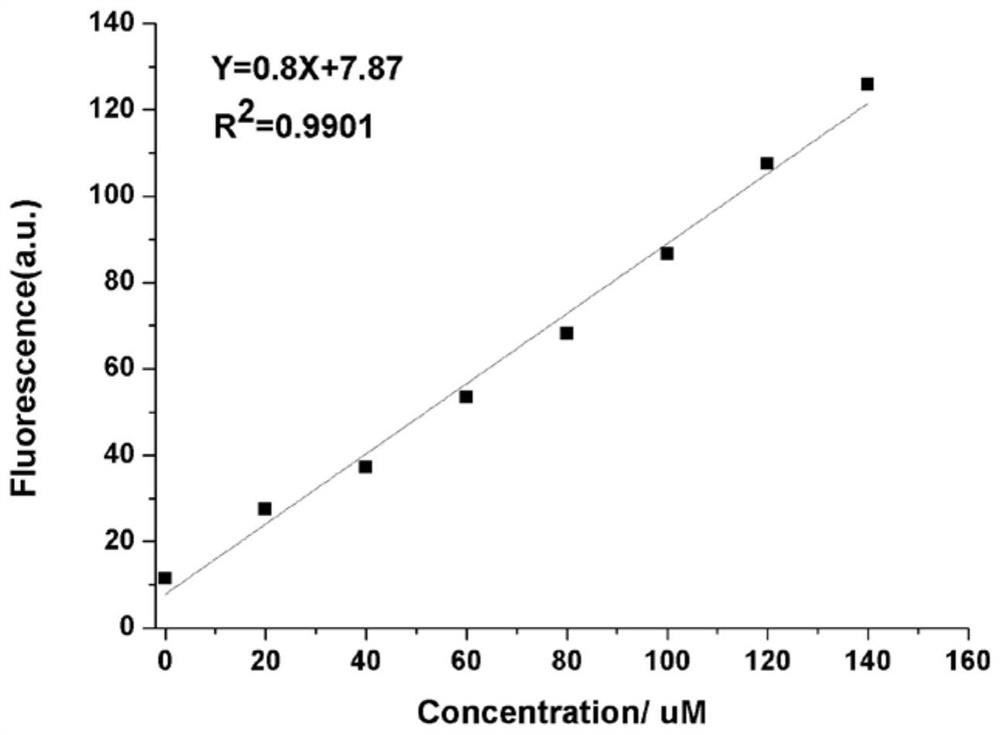
[0040] Example 2 Responses of dithiothreitol fluorescent probes to different concentrations of dithiothreitol.
[0041] Dissolve the dithiothreitol fluorescent probe prepared in Example 1 in methanol, and then prepare a 10 μM probe solution with phosphate buffered saline (pH 7.4). The methanol content in the solution is 20wt%; Thiothreitol solution, the concentration of which is 0, 10, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240, 260, 280, 300 μM; The probe solution was mixed with different concentrations of dithiothreitol solutions for 30 min for fluorescence detection (λ ex =580nm, λ em = 638nm), measure the fluorescence intensity in each system.
[0042] Taking the wavelength as the abscissa and the fluorescence intensity as the ordinate, the response of the fluorescent probe to different concentrations of dithiothreitol is as follows: figure 2 Shown: the peak value of the fluorescence intensity is around 638nm, as the concentration of thiothreitol increases,...
Embodiment 3
[0043] Example 3 Effects of different reaction times on the fluorescence intensity of the dithiothreitol fluorescent probe.
[0044] Dissolve the dithiothreitol fluorescent probe prepared in Example 1 in methanol, and then prepare a 10 μM probe solution with phosphate-buffered saline (pH 7.4), in which the methanol content is 20wt%; 300 μM dithiothreitol solution was prepared in buffered saline (pH 7.4). Then 5 mL of fluorescent probe solution was mixed with 300 μM dithiothreitol solution for 30 min for fluorescence detection (λ ex = 580nm, λ em = 638nm), the fluorescence intensity in the system was measured every 2min for 40min.
[0045] Taking the wavelength as the abscissa and the fluorescence intensity as the ordinate, the fluorescence intensity of the fluorescent probe to dithiothreitol with different reaction times is as follows: Figure 4 Shown: the peak value of the fluorescence intensity is around 638nm, with the prolongation of the reaction time, the peak value of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com