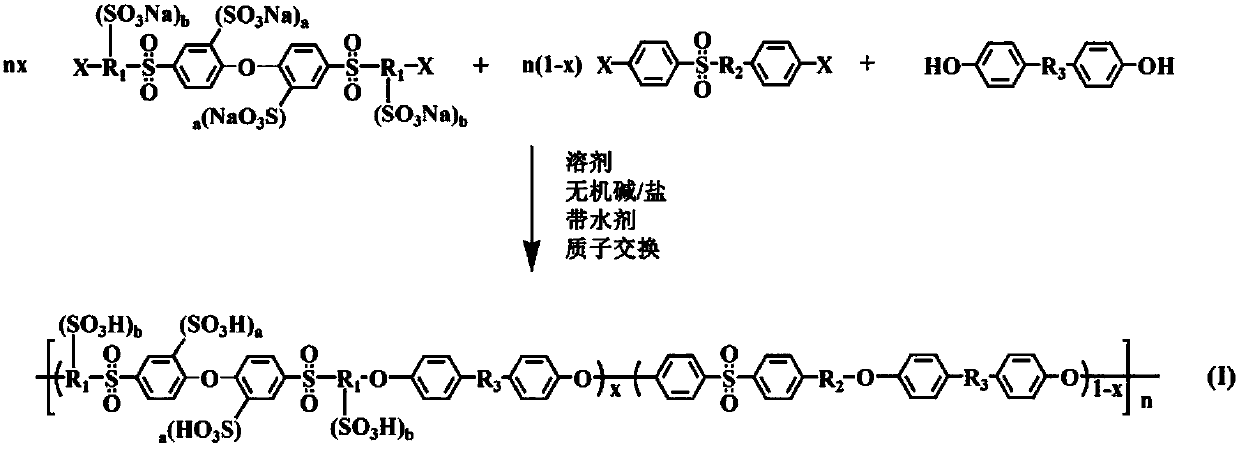
Ether bond bridged polysulfone sulfonated polyether sulfone, and preparation method and application thereof
A technology for sulfonating polyethersulfone and polysulfone groups, which is applied in the field of polymer materials, can solve the problems of few sulfonic acid groups and single structure, and achieve the effects of simple preparation process, easy structure adjustment, and high proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
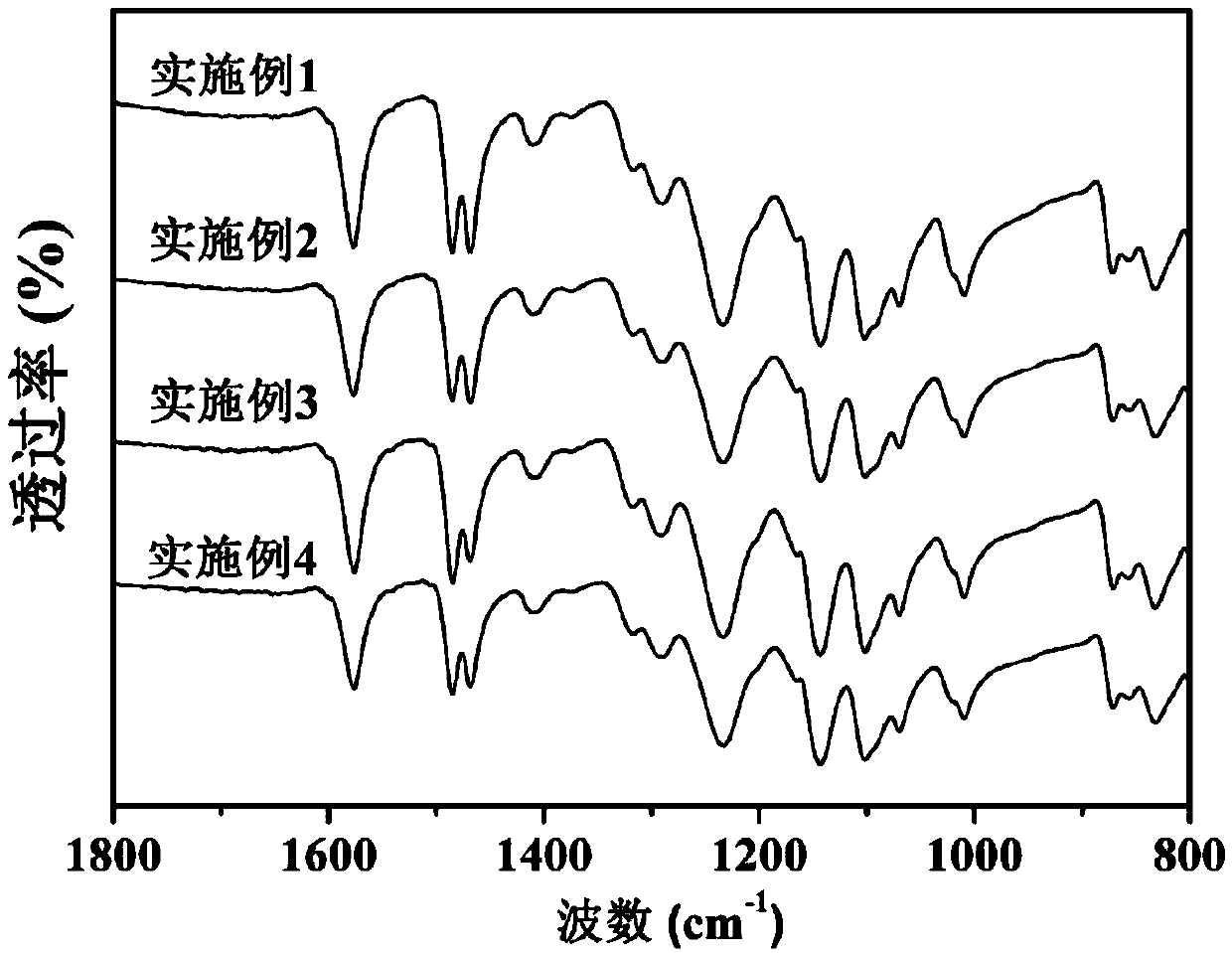
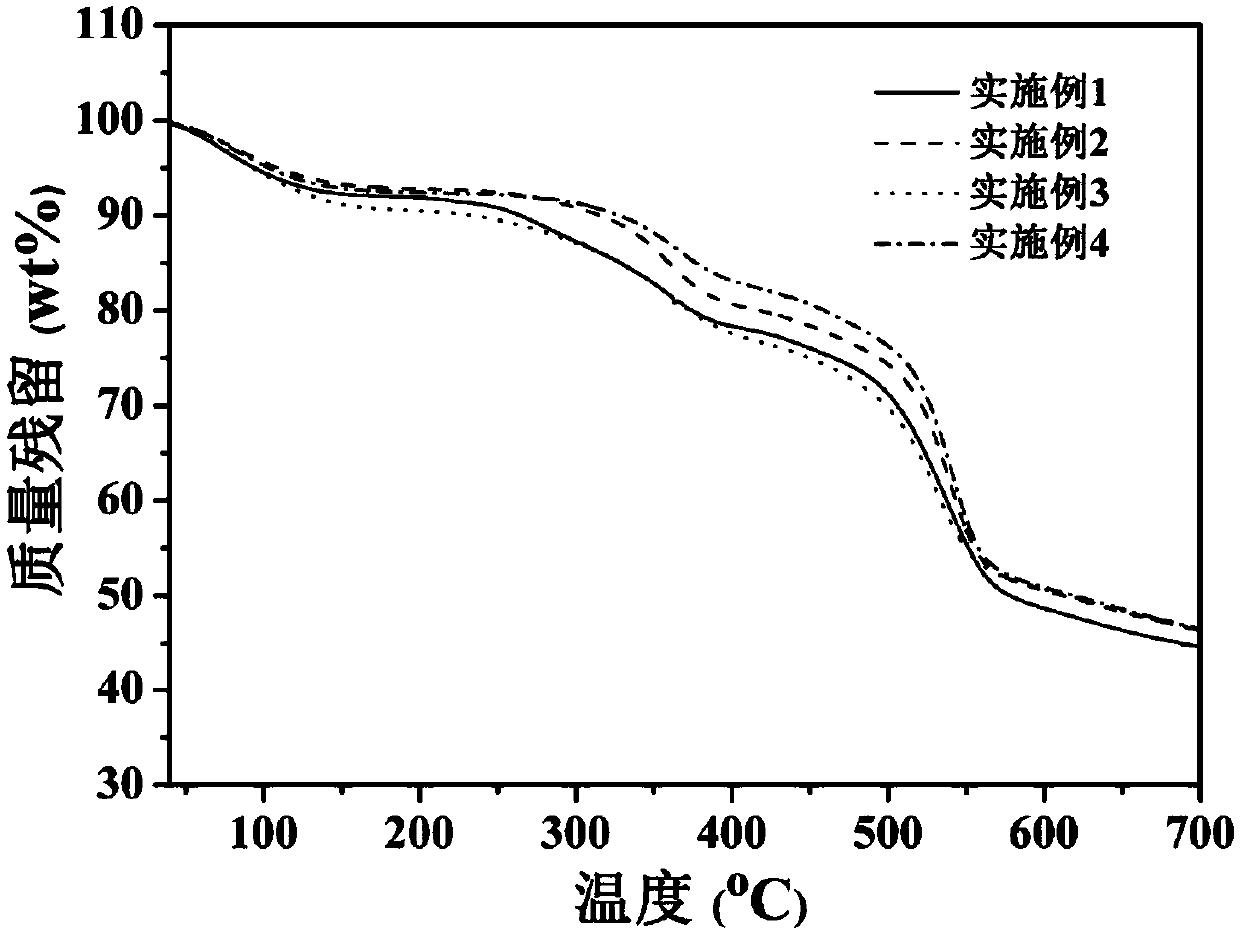
Embodiment 1
[0036] Example 1: Synthesis of polysulfone group sulfonated polyethersulfone homopolymer Hp-1 bridged by ether bonds and preparation of proton exchange membrane
[0037] Under nitrogen protection and mechanical stirring, add 4,4'-bis(4-fluorobenzenesulfonyl-3-sodium sulfonate)diphenyl ether-2,2 17.88g (20mmol) of '-sodium disulfonate, 3.72g (20mmol) of 4,4'-diphenol, and 70-100mL of N,N-dimethylacetamide, preferably, the optimal addition of this example The volume is 90 mL. After the solid is completely dissolved, add 5.52-6.90g (40-50mmol) of potassium carbonate and 10-20mL of toluene. Preferably, the optimal addition amounts in this embodiment are 6.07g (44mmol) and 15mL respectively. After stirring evenly, raise the temperature of the reaction system to 120-140°C for 2-6 hours, evaporate the water generated in the reaction, and then raise the temperature to 140-170°C for 8-24 hours. Preferably, the two best The temperature points are 140°C and 160°C, and the two times are...
Embodiment 2
[0039] Example 2: Synthesis of polysulfone group sulfonated polyethersulfone homopolymer Cp-1-4 / 1 / 3 bridged by ether bonds and preparation of proton exchange membrane
[0040] Under nitrogen protection and mechanical stirring, add 4,4'-bis(4-fluorobenzenesulfonyl-3-sodium sulfonate)diphenyl ether-2,2 8.94g (10mmol) of '-sodium disulfonate, 7.62g (30mmol) of 4,4'-difluorodiphenyl sulfone, 7.44g (40mmol) of 4,4'-biphenyldiphenol and N,N-dimethyl Acetamide 80-120mL, preferably, the optimal addition amount of this embodiment is 100mL. After the solid is completely dissolved, 11.04-13.80 g (80-100 mmol) of potassium carbonate and 12-25 mL of toluene are added. Preferably, the optimal addition amounts in this embodiment are 11.59 g (84 mmol) and 18 mL respectively. After stirring evenly, raise the temperature of the reaction system to 120-140°C for 2-6 hours, evaporate the water generated in the reaction, and then raise the temperature to 140-170°C for 8-24 hours. Preferably, the t...
Embodiment 3
[0042] Example 3: Synthesis of polysulfone group sulfonated polyethersulfone homopolymer Cp-1-3 / 1 / 2 bridged by ether bonds and preparation of proton exchange membrane
[0043] Under nitrogen protection and mechanical stirring, add 4,4'-bis(4-fluorobenzenesulfonyl-3-sodium sulfonate)diphenyl ether-2,2 8.94g (10mmol) of '-sodium disulfonate, 5.08g (20mmol) of 4,4'-difluorodiphenyl sulfone, 5.58g (30mmol) of 4,4'-biphenyldiphenol and N,N-dimethyl Acetamide 65-98mL, preferably, the optimal addition amount of this embodiment is 80mL. After the solid is completely dissolved, add 8.28-10.35g (60-75mmol) of potassium carbonate and 8-16mL of toluene. Preferably, the optimal addition amounts in this embodiment are 8.83g (64mmol) and 12mL respectively. After stirring evenly, raise the temperature of the reaction system to 120-140°C for 2-6 hours, evaporate the water generated in the reaction, and then raise the temperature to 140-170°C for 8-24 hours. Preferably, the two best The tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com