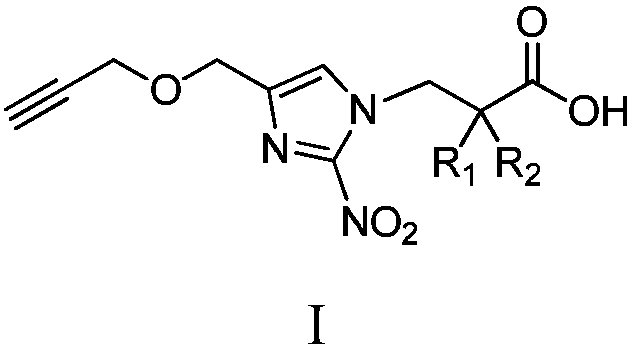
Branched 2-nitroimidazole compound and application thereof in hypoxic selective antitumor prodrugs
A technology for nitroimidazoles and antitumor drugs, applied in the field of compounds, can solve problems such as insufficient targeting and poor effect, and achieve the effects of improving targeting, reducing toxic and side effects, enhancing curative effect and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of compound 3
[0040]
[0041] Cesium carbonate (6.52g, 20mmol) and compound 1 (1.13g, 10mmol) were dissolved in 15mL of N,N-dimethylformamide, the mixture was stirred at room temperature for 15 minutes, and compound 2 (5.12g, 20mmol) was added. Heated to 80°C, stirred overnight, and the reaction was complete. Quenched by adding water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated the organic phase, and separated by column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain 2.4 g of yellow oil with a yield of 99%. 1 H NMR (400MHz, CDCl 3 )δ7.13(s, 1H), 7.11(s, 1H), 4.74(s, 2H), 4.15(q, J=7.2Hz, 2H), 1.25(t, J=6.7Hz, 3H), 1.21( d,J=5.8Hz,6H).MS(ESI) Calcd for C 10 h 16 N 3 o 4 [M+H] + :242.1, found: 242.1.
Embodiment 2
[0043] Preparation of Compound 4
[0044]
[0045] Compound 3 (4 g, 16.6 mmol) was dissolved in 20 mL of DMF, NBS (5.9 g, 33.2 mmol) was added, and stirred overnight at room temperature. After the reaction was complete, it was quenched by adding water, extracted with ethyl acetate, and dried over anhydrous sodium sulfate. The organic phase was concentrated and separated by column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 3.5 g of a yellow solid with a yield of 66% and a melting point of 65-67°C. 1 H NMR (400MHz, CDCl 3 )δ7.17(s,1H),4.72(s,2H),4.16(q,J=7.1Hz,2H),1.26(t,J=7.2Hz,4H),1.23(s,6H). 13 CNMR (100MHz, CDCl 3 )δ175.4, 126.2, 115.1, 61.9, 55.6, 44.7, 23.1, 22.2, 14.1. HRMS (ESI): m / z Calcd for C 10 h 14 N 3 BrNa[M+Na] + :342.0006,found:342.0071.
Embodiment 3
[0047] Preparation of Compound 6
[0048]
[0049]Under nitrogen protection, compound 4 (1g, 3mmol), Pd(dppf)Cl 2 DCM (245mg, 0.3mmol), potassium phosphate (1.27g, 6mmol) were suspended in 10mL DMF, compound 5 (0.92g, 6mmol) was added dropwise, heated to 60°C, and stirred overnight. Some raw materials could not react completely, quenched by adding water, extracted with ethyl acetate, dried over anhydrous sodium sulfate. After the organic phase was concentrated, it was separated by column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain 0.5 g of a yellow solid with a yield of 62% and a melting point of 58-60°C. 1 H NMR (400MHz, CDCl 3 )δ7.10(s,1H),6.53(dd,J=17.5,11.0Hz,1H),5.94(d,J=17.5Hz,1H),5.32(d,J=11.0Hz,1H),4.70( s,2H), 4.14(q,J=7.1Hz,2H),1.26–1.21(m,9H). 13 C NMR (100MHz, CDCl 3 )δ175.5, 139.1, 126.9, 123.3, 116.7, 61.6, 55.4, 44.6, 23.0, 22.1, 14.1. HRMS (ESI): m / z Calcd for C 12 h 17 N 3 o 4 Na[M+Na] + :290.1111,found:290.1128.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com