Temozolomide injectable composition and preparation method thereof
A technology for temozolomide and composition, which is applied in the field of temozolomide injectable composition and its preparation field, can solve the problems of potential safety hazards for patients, long reconstitution time and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] The present invention also provides a preparation method of the temozolomide composition of the present invention, comprising the steps of:
[0078] 1) Dissolve other excipients except the active ingredient in water for injection, and adjust the pH to 2.5-5.0;
[0079] 2) Add the active ingredient, stir to dissolve, and constant volume;
[0080] 3) filter;
[0081] 4) Packing;
[0082] 5) freeze-drying.
[0083] Preferably, the preparation method comprises the following steps:
[0084] 1) Dissolve glycine, sodium sulfobutylbeta cyclodextrin, nonionic surfactant, buffer, and lyoprotectant in water for injection, add a pH regulator to adjust the pH to 3.5-4.5, and control the temperature of the solution 30°C or below (such as 5-29°C or 15-29°C);
[0085] 2) adding temozolomide or a pharmaceutically acceptable salt thereof, stirring to dissolve, and constant volume;
[0086] 3) sterile filtration;
[0087] 4) sub-package injection bottles;
[0088] 5) freeze-drying...
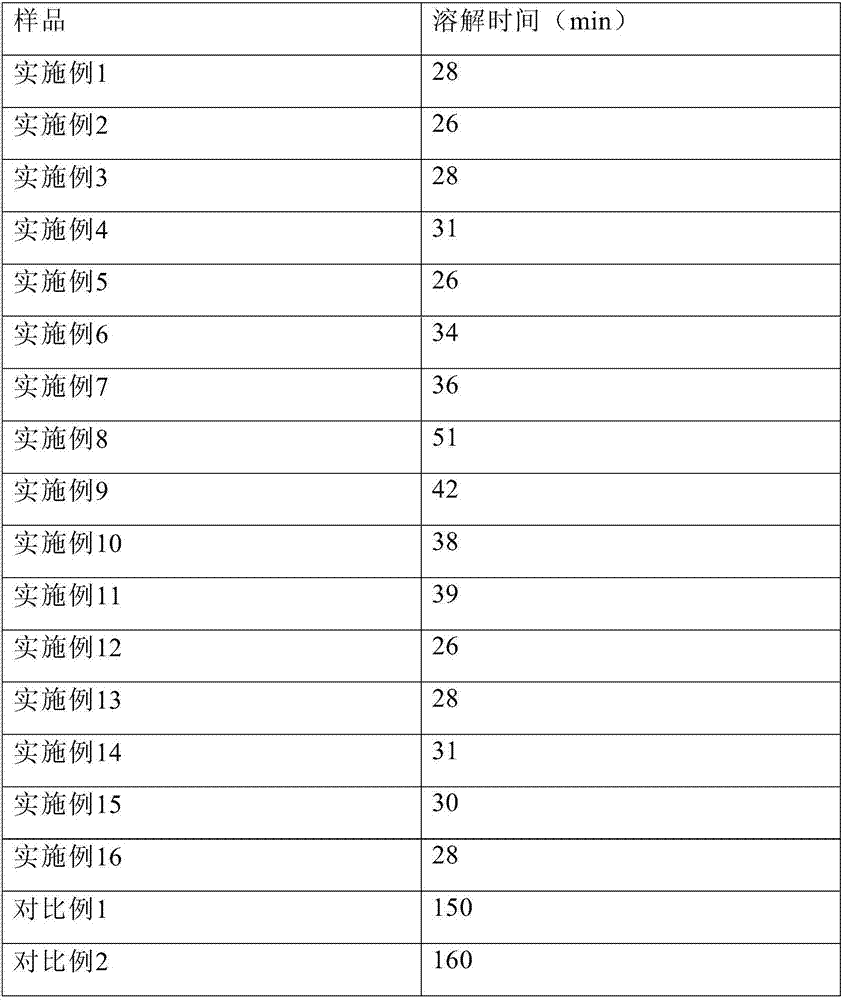
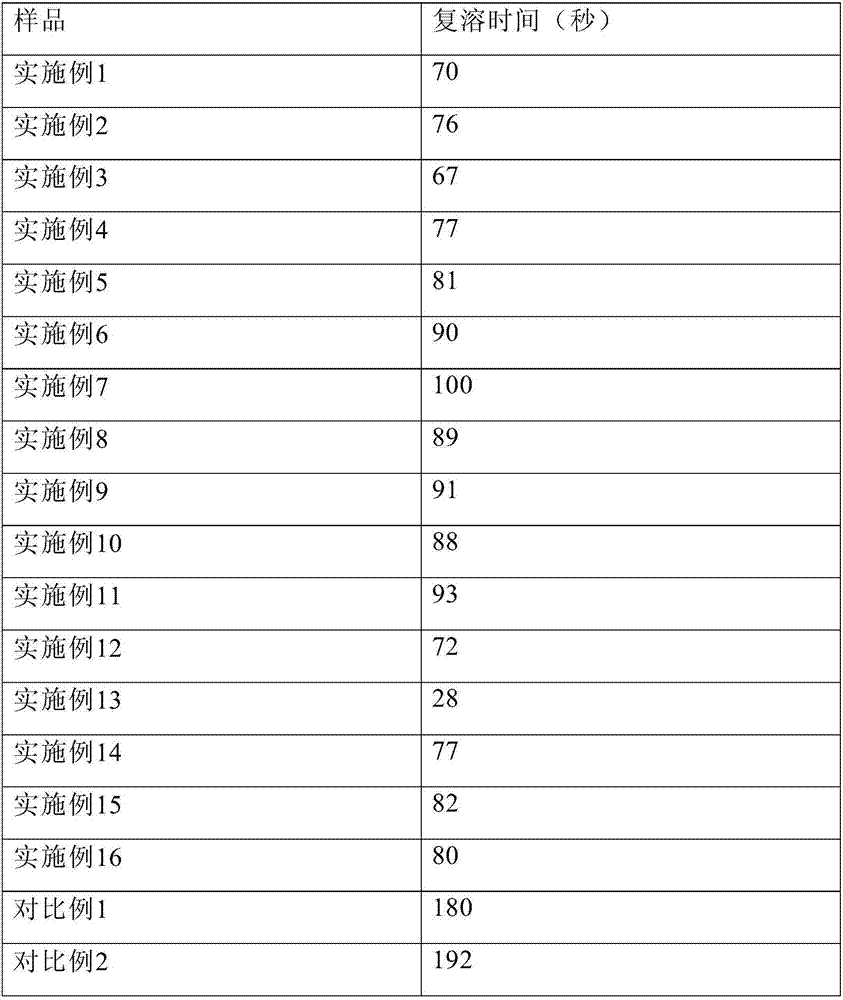
Embodiment 1
[0104] Dissolve 12.0 g of glycine, 4.0 g of sodium sulfobutylbeta cyclodextrin, 12.0 g of Tween 80, 20.0 g of sodium citrate, and 60.0 g of mannitol in 3.2 L of water for injection, and add about 13.0 g of hydrochloric acid to adjust the pH to 3.8. Control the solution temperature to 20°C. Add 10.0 g of temozolomide, stir to dissolve, dilute to 4 L, filter aseptically, divide 40 ml / bottle into injection vials, lyophilize, stopper, and seal.
Embodiment 2
[0106] Dissolve 15.0g glycine, 1.0g sodium sulfobutylbeta cyclodextrin, 15.0g polyethylene glycol hydroxystearate, 10.0g sodium citrate, and 120.0g mannitol in 3.2L water for injection, and add about 6.0 g of hydrochloric acid was used to adjust the pH to 4.0. Control the solution temperature to 25°C. Add 10.0 g of temozolomide, stir to dissolve, dilute to 4 L, filter aseptically, divide 40 ml / bottle into injection vials, lyophilize, stopper, and seal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com